The best of both worlds: Basic-to-acidic flash switching for organic synthesis
Scientists at Tokyo Institute of Technology developed a fast and practical technique using a micro-flow reactor for the synthesis of pure N-carboxy anhydrides (NCAs). They exploited the advantages of carrying out the synthesis under basic conditions and avoided its disadvantages by flash switching to acidic conditions after 0.1 seconds. This approach enabled them to produce several types of NCAs in a scalable, space-saving, and less time-consuming fashion.
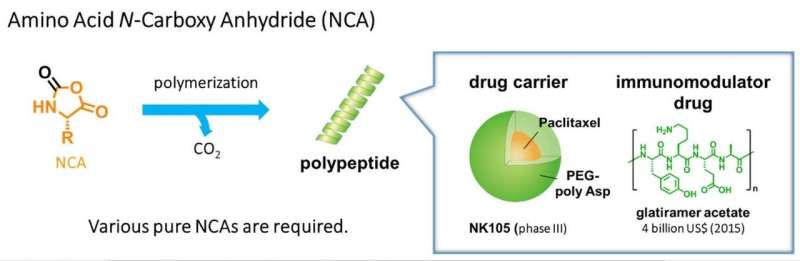
The synthesis of polypeptides is of enormous importance in many applications, including life-saving drugs and drug carriers. In turn, the synthesis of pure N-carboxy anhydrides (NCAs) is essential for the primary process used to prepare polypeptides, which is the polymerization (chaining) of NCAs (see Figure 1). However, the only practical method for synthesizing NCAs was established in 1922, called the Fuchs-Farthing method.
This old but effective method evidently has its limitations. It requires harsh acidic conditions and can cause undesired ring opening in the NCAs produced. In this one-step method, amino acids are mixed with phosgene to obtain the desired NCAs, along with HCl and CO2, in a slow (two to five hours) but stable fashion with some side reactions, which decrease the purity of the final product.
One the other hand, synthesizing NCAs under basic conditions is a no go because, even though NCAs are formed very quickly in such conditions, the deprotonation of their NH groups causes them to polymerize, making this approach unfeasible. Associate Professor Fuse, a member of a team of scientists from Tokyo Institute of Technology who worked on this topic, explains: "The desired reaction proceeds smoothly under basic conditions; however, basic conditions induce undesired polymerization of the product."
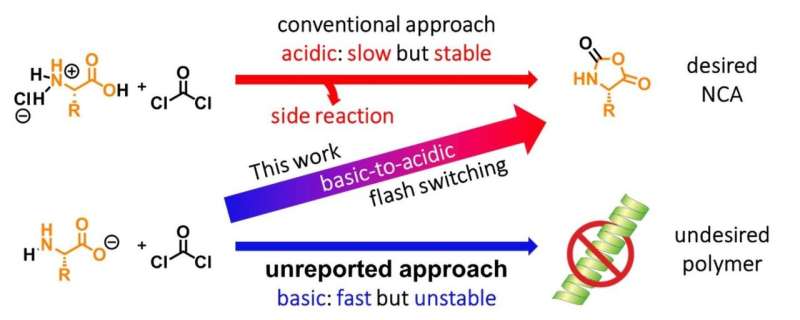
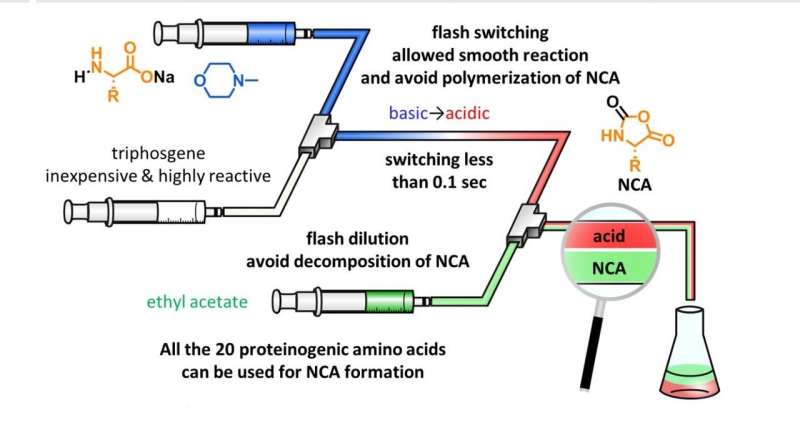
How did the team solve this problem of organic synthesis? They inferred that they could have the necessary reactions take place in basic conditions first (which happen in less than 0.1 seconds) and then quickly change the pH to acidic to prevent undesired polymerization (see Figure 2). This pH flash switching was accomplished using the scheme shown in Figure 3. The team employed a micro-flow reactor to be able to mix the amino acids with triphosgene in less than 0.1 seconds. Then, by excessively adding triphosgene, the HCl produced quickly changes the pH from basic to acidic. Finally, to prevent the acidic decomposition of acid-labile NCAs, they quickly diluted with ethyl acetate. Using this technique, the team managed to synthesize NCAs using all the proteinogenic amino acids and several acid-labile nonproteinogenic amino acids.
NCAs are very important and unstable materials, and have to be stored and transported under strictly controlled temperatures. This has unfortunately limited their use. However, because micro-flow synthesis is an easily scalable and space-saving approach, the proposed method could pave the way for on-demand and on-site synthesis of NCAs.
More information: Yuma Otake et al, Rapid and Mild Synthesis of Amino Acid N -Carboxy Anhydrides: Basic-to-Acidic Flash Switching in a Microflow Reactor, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201803549
Journal information: Angewandte Chemie International Edition
Provided by Tokyo Institute of Technology