Investigating new materials to reduce the operation temperature of solid oxide fuel cells

Researchers at TU Wien are investigating new materials that can be used to reduce the operation temperature of solid oxide fuel cells. To do so, they apply an innovative method.
Solid oxide fuel cells consist of three important parts: an anode, a cathode and an electrolyte. While oxygen is incorporated into the cathode, oxygen is then transported through the electrolyte to the anode, where oxygen reacts with hydrogen to water. The fuel cell is able to convert the energy released in the process into electricity. For this reason, fuel cells are increasingly used in stationary energy supply and in the automotive industry.
In order to reduce the operating temperature of solid oxide fuel cells from currently 800 °C to 450 °C to 600 °C, scientists at TU Wien are researching alternative materials that are suitable to serve as cathodes at this lower temperatures. Markus Kubicek and his team recently published the results of their material analysis in the Journal of Materials Chemistry A.
Reduced operation temperature
Solid oxide fuel cells have been built since the 1980s. Now researchers are trying to develop new fuel cells that offer high long term stability and are cheaper to fabricate. To do so, it is necessary to lower the operating temperature to about 450 °C to 600 °C. For the operation of the solid oxide fuel cell at lower temperatures, especially the rather slow oxygen exchange at the cathode represents a bottleneck. Researchers worldwide are therefore searching for ways to develop new electrode materials that can incorporate oxygen sufficiently fast even at those lower temperatures.
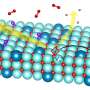
Oxygen exchange pathways
Scientists in the research division "Technical Electrochemistry" have been working on so-called mixed conducting materials (MIECs) for years. Oxides of this class of materials are particularly well suited for fuel cell cathodes, as they can conduct both oxygen ions and electrons at higher temperatures. This works primarily via defects, i.e. minimal deviations from the ideal crystal lattice, which are intentionally introduced into the material.
"The most important defects inside these materials are oxygen vacancies, electrons and holes. In order to be able to optimize these materials in a targeted manner, a better understanding of the role of these defects for the oxygen incorporation reaction is of high importance," explains Markus Kubicek, head of the FWF project "In-Situ Characterisation of Oxidic Thin Films During Growth." The researchers have now succeeded in doing that.
Unique measuring technique worldwide
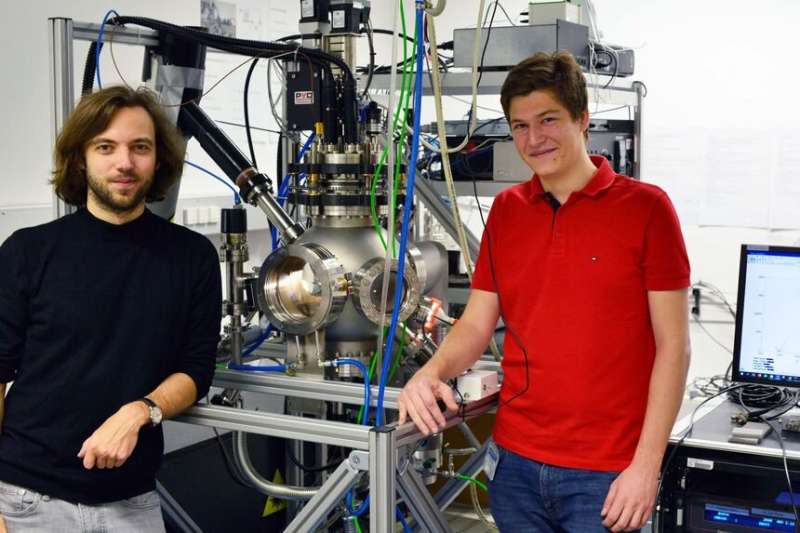
To measure the kinetics of the oxygen incorporation reaction, the researchers use "in situ PLD" measurements, which are unique worldwide. The electrode materials are deposited in a vacuum chamber with a laser and are measured directly after deposition applying impedance spectroscopy. "Since even the smallest impurities can have a strong influence on the measurement results, we needed a measurement method which allowed us to examine pristine electrode surfaces. We succeeded in doing that here for the first time," explains Christoph Riedl research group of solid state ionics. "Only through our in-situ method developed here were we able to perfectly combine theoretical simulation and real measurement results," he adds.
Different materials, same oxygen pathways
The researchers used their measurement method to investigate the oxygen exchange reaction on the surface of five promising materials. "A highlight of our measurements is that for the first time we were able to observe that the oxygen exchange reaction seems to follow the same mechanism on very different materials," describes Matthäus Siebenhofer. "A decisive factor here is the availability of oxygen vacancies on the surface."
Jürgen Fleig, head of the working group "Solid State Ionics," concludes: "In this study, we were able to combine various research results and experimental developments of the last few years and thus describe and understand the most important reaction in the field of solid oxide fuel cells much better."
More information: Matthäus Siebenhofer et al, Investigating oxygen reduction pathways on pristine SOFC cathode surfaces by in situ PLD impedance spectroscopy, Journal of Materials Chemistry A (2021). DOI: 10.1039/D1TA07128A
Journal information: Journal of Materials Chemistry A
Provided by Vienna University of Technology