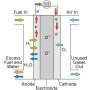
How does oxygen get into a fuel cell?
In order for a fuel cell to work, it needs an oxidizing agent. TU Wien has now found a way to explain why oxygen does not always enter fuel cells effectively, rendering them unusable.
Fuel cells use a simple chemical reaction, such as the combination of oxygen and hydrogen to form water, to generate electricity. The question of which is the best material to use when making ceramic fuel cells is not a straightforward one, however. New materials are required that act as a catalyst for the chemical reaction required with maximum efficiency, but that also last as long as possible without their properties changing.
Previous efforts to develop materials that fulfil these requirements have largely been based on trial and error. However, teams at TU Wien have now managed to find a way to make targeted alterations to the surface of fuel cells on an atomic scale and take measurements at the same time. As a result, it is now possible to explain important phenomena for the first time, including the reasons why strontium atoms are problematic and the fact that cobalt can be useful in a fuel cell.
The oxygen supply bottleneck
At the cathode, the positive terminal of the fuel cell, oxygen is incorporated into the fuel cell material from the air. Electrically charged oxygen ions then have to travel through the material and react with the fuel, for example hydrogen, at the negatively charged side, the anode.
"The bottleneck within this whole process is the incorporation of oxygen at the cathode," explains Ghislain Rupp, from the research group led by Professor Jürgen Fleig at TU Wien's Institute of Chemical Technologies and Analytics. The team led by Professor Andreas Limbeck and based at the same institute was also involved in this research project.
Fuel cells need to be operated at extremely high temperatures, somewhere in the region of between 700 and 1000 degrees Celsius, in order to ensure that the oxygen is incorporated quickly enough. Researchers have long been trying to identify better cathode materials that will allow for the operating temperature to be reduced. "There are some well-known options that are of particular interest, including lanthanum strontium cobaltite, or LSC for short," explains Ghislain Rupp. The major problem in this case is that these materials do not remain stable in the long term. There is always a point at which the activity drops and the performance of the fuel cell dwindles. Until now, it has only been possible to guess at the precise reason for this.
Targeted surface alterations
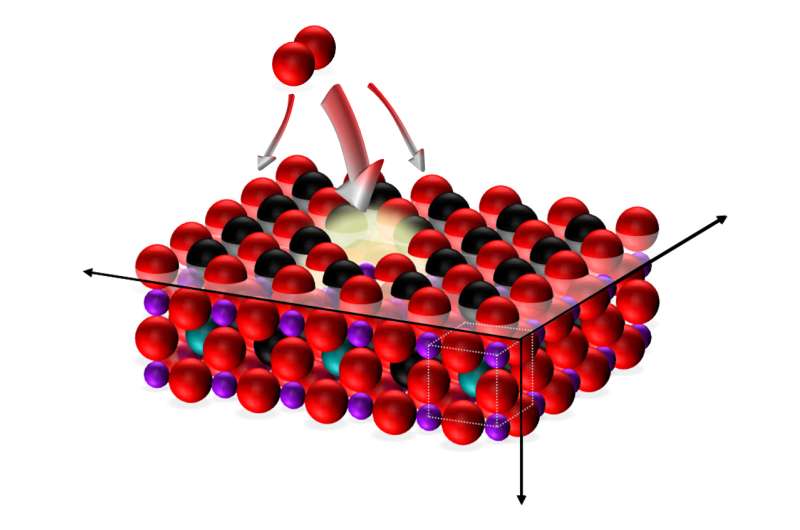
One thing has always been clear: the surface of the cathode, where the oxygen is to settle before entering the fuel cell, has a crucial role to play. With this in mind, the teams at TU Wien developed a method of making targeted alterations to the surface that also allows for measurements to be taken at the same time in order to determine the effects of this on the electrical properties of the fuel cell.
"We use a laser pulse to vaporise various materials, which then accumulate in tiny volumes on the surface," explains Rupp. "This enables us to modify the composition of the cathode surface in small, precise doses, whilst also monitoring how this affects the resistance of the system."
The damaging effect of excessive strontium
In this way, we have been able to show that materials containing large volumes of strontium on the surface have a damaging effect: "If there are too many strontium atoms on the surface, oxygen is not incorporated very effectively at all," says Rupp. "The oxygen is taken up by the cathode surface very unevenly. At some preferential spots, for example where cobalt atoms are located, oxygen is incorporated effectively. However, at the points where strontium dominates, hardly any oxygen is able to enter the cathode." This also explains why the fuel cells deteriorate over time, as the strontium inside the material migrates to the surface and covers any active cobalt accumulations, ultimately keeping the air away from the fuel cell.
These findings provide important information on how oxygen is fundamentally incorporated into materials such as LSC and which processes cause the performance of fuel cells to deteriorate. "This research has taken us a huge step closer to the technical use of LSC as a fuel cell material," Rupp believes. "What's more, our new method of investigation combining ultra-precise coating with electrical measurement is sure to find other important applications within the field of solid state ionics."
More information: Ghislain M. Rupp et al, Real-time impedance monitoring of oxygen reduction during surface modification of thin film cathodes, Nature Materials (2017). DOI: 10.1038/nmat4879
Journal information: Nature Materials
Provided by Vienna University of Technology