This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Protons can tune synaptic signaling by changing the shape of a protein receptor
Synaptic plasticity, the ability of synapses to strengthen or weaken over time in response to changes in their activity, is a central foundation of learning and memory. At synapses, presynaptic cells transmit signals to postsynaptic cells through a dance that is orchestrated by neurotransmitters, protons, receptors, scaffolding proteins, signaling molecules, and more.
AMPA receptors, ligand-gated ion channels that mediate fast, excitatory synaptic transmissions, are activated by glutamate, the primary neurotransmitter of the mammalian brain. Surprisingly, however, AMPARs have a low affinity for glutamate, so they anchor themselves in close physical proximity to the site of glutamate release for optimal activation. The anchoring is mediated in part by their N-terminal domains.
During synaptic transmission, some protons are released together with glutamate, and the co-released protons participate in synaptic signaling mediated by the AMPARs. However, how protons modify AMPAR signaling has been unknown.
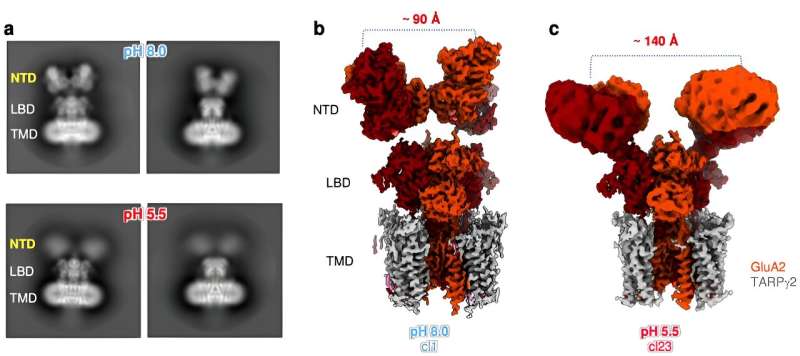
Now, research from the labs of Teru Nakagawa, professor of molecular physiology and biophysics, and Ingo Greger, group leader at the MRC Laboratory of Molecular Biology in Cambridge, England, reveals that a brief decrease in pH—a brief increase in the concentration of protons—can lead to changes in AMPAR's structure, thereby modulating its location and gating kinetics.
Certain health conditions, such as ischemia (reduced blood flow and oxygenation) and stroke, are known for creating acidic environments in the brain, so understanding the role of protons on neuronal function can lead to a more nuanced understanding of brain injury and recovery.
Nakagawa's new research, published in Nature Structural & Molecular Biology, shows that when protons are released at the synapse and interact with a particular amino acid in an AMPAR, they cause the receptor's N-terminal domain to splay in half. The effects of the splaying are two-fold: It slows the receptor's recovery before it can activate again and it increases receptor diffusion by breaking the receptor's anchor from the optimal location for activation, ultimately reducing AMPAR activity and impacting synaptic strength and plasticity.
The molecular processes behind cognition, learning, and memory formation are not well understood, yet scientists know that AMPARs are central to these processes. In addition to their role in normal physiology, deficiencies in AMPAR functioning have been linked to a variety of neurological and psychiatric disorders such as seizures, Alzheimer's disease, major depressive disorder, limbic encephalitis, intellectual disability, and autism spectrum disorder.
Nakagawa's work on resolving the role of protons during synaptic transmission will have particular implications for our understanding of short-term and long-term synaptic plasticity.
More information: Josip Ivica et al, Proton-triggered rearrangement of the AMPA receptor N-terminal domains impacts receptor kinetics and synaptic localization, Nature Structural & Molecular Biology (2024). DOI: 10.1038/s41594-024-01369-5
Journal information: Nature Structural & Molecular Biology