New catalyst may hasten commercialization of fuel cell vehicles
Scientists at the U.S. Department of Energy's Argonne National Laboratory have developed a new fuel cell catalyst using earthly abundant materials with performance that is comparable to platinum in laboratory tests. If commercially viable, the new catalyst could replace platinum in electric cars powered by fuel cells instead of batteries, which would greatly extend the range of electric vehicles and eliminate the need for recharging.
Fuel cells generate electricity by using hydrogen from a fuel tank with oxygen in the air. The only waste product emitted to the environment is water.
But fuel cells are expensive, largely because they depend on the precious metal platinum to cause the hydrogen-oxygen reaction. Argonne's fuel cell catalyst replaces much of the platinum with a non-precious metal.
"Platinum represents about 50 percent of the cost of a fuel cell stack, so replacing or reducing platinum is essential to lowering the price of fuel cell vehicles," said Di-Jia Liu, who led the Argonne team. Their catalyst replaces all the platinum in the fuel cell's cathode, which usually requires four times as much platinum as the anode, and their new electrode design also optimizes the flow of protons and electrons within the fuel cell and the removal of water.
Many automakers see sales of vehicles powered by fuel cells as eventually outpacing battery-powered electric vehicles for several reasons: fuel-cell vehicles emit only water, can travel over 300 miles between fill ups, can be refilled quickly and place no burden on the electrical grid because they don't need recharging.
Since both technologies lack refilling or recharging infrastructures and are expensive, both are currently suitable mainly for early adopters and use in corporate fleets. But this may change, if advances made by Argonne researchers can be realized in commercial fuel-cell vehicles.
Fuel cells generate electricity to propel vehicles through electrochemical reactions between onboard hydrogen fuel and oxygen in the air. Hydrogen molecules are stripped of electrons at the fuel cell's anode, becoming protons that travel through a polymer electrolyte membrane to the cathode, where they react with electrons and oxygen to form water.
"In order for a fuel cell to work," Liu explained, "the catalyst must be densely packed with active sites that are uniformly distributed throughout the cathode and directly connected to the arriving protons and electrons, while maintaining easy access to oxygen. The catalyst should also have an architecture that can readily channel away the produced water." No conventional method for preparing carbon-based platinum or non-precious metal catalysts can meet all these criteria, Liu added.
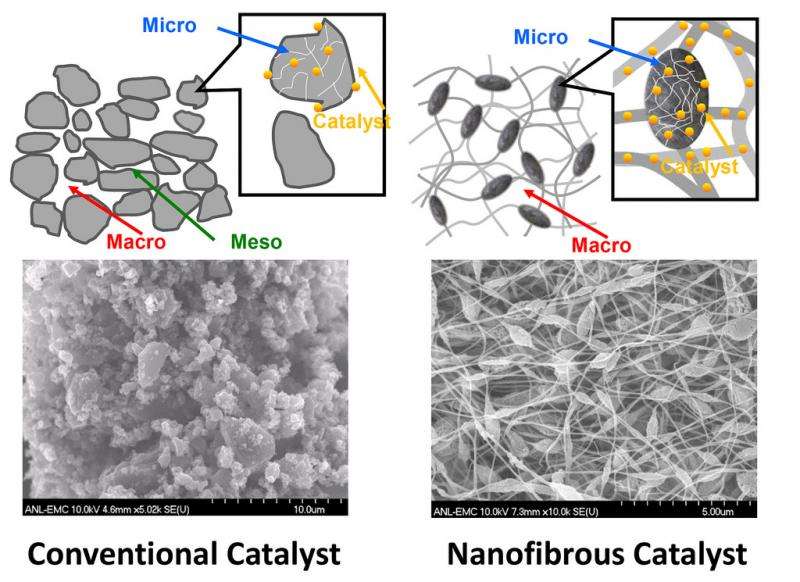
In a paper recently published in the Proceedings of the National Academy of Sciences of the United States of America, the team led by Liu reported on a new method of synthesizing a highly efficient, nanofibrous non-precious metal catalyst by electrospinning a polymer solution containing a mixture of ferrous organometallics and metal-organic frameworks. Following thermal activation, the new catalyst delivered an unprecedented level of catalytic activity in actual fuel cell tests.
"The new catalyst offers a unique carbon nano-network architecture made of microporous nanofibers interconnected through a macroporous framework," Liu explained. "Not only do the active sites inside the micropores within individual fibers catalyze chemical reactions effectively, but the macroporous voids between the fibers transport oxygen and water efficiently to and from the active sites. The continuous nano-networks also make the catalytic electrode highly conductive in charge transfer."
More information: "Highly efficient nonprecious metal catalyst prepared with metal–organic framework in a continuous carbon nanofibrous network." PNAS 2015 112 (34) 10629-10634; published ahead of print August 10, 2015, DOI: 10.1073/pnas.1507159112
Journal information: Proceedings of the National Academy of Sciences
Provided by Argonne National Laboratory