Novel catalyst design opens possibility to hydrogen vehicle

A new research, affiliated with UNIST has presented a novel strategy for non-precious metal catalyst that can replace rare and expensive platinum(Pt)-based catalyst, currently used in hydrogen fuel cell.
In their study, published in the November issue of the Journal of the American Chemical Society, Professor Sang Hoon Joo of Energy and Chemical Engineering and his team have devised a new synthetic strategy to boost the activity of iron- and nitrogen-doped carbon (Fe-N/C) catalyst that can realize low-cost hydrogen fuel cell.
Hydrogen fuel cell generates electricity with hydrogen and oxygen, producing water as a byproduct. Precious platinum(Pt) has been used in commercialized fuel cell. However, the high cost of Pt (>40$ per g) hampers widespread application of the fuel cell.
The research team has attempted to develop high-performance non-precious metal catalyst which can substitute for state-of-the-art Pt-based catalysts. In this research, they focused on carbon-based catalyst with iron and nitrogen due to low cost and high activity (Fe-N/C catalyst). During the preparation of the Fe-N/C catalysts, high-temperature heat-treatment at over 700 ? is commonly required to endow high catalystic activity, but unfortunately this treatment also diminishes the number of active site. The active site refers to the place where rate-determining catalytic reaction occurs.
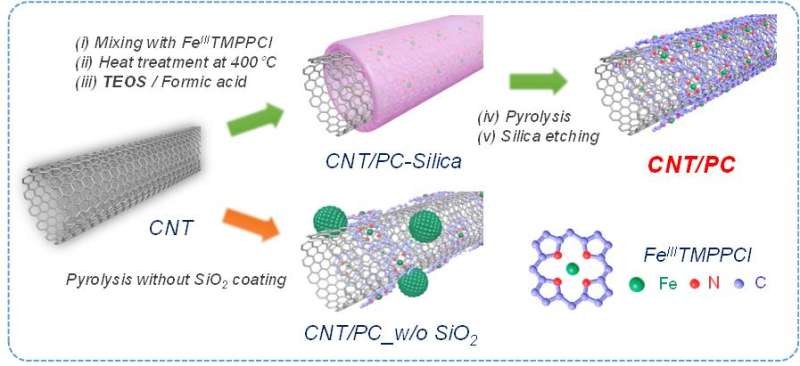
To solve the problem, they have introduced 'silica-protective-layer' approach. The silica layer effectively preserved the active site at high-temperature, preventing the destruction of the active site.
The novel Fe-N/C catalyst prepared by 'silica-protective-layer' approach showed very high oxygen reduction reaction (ORR) activity which is comparable to Pt catalyst. ORR is an electrochemical reaction at the cathode of hydrogen fuel cell. Due to 1-million-times slower reaction kinetics of ORR at the cathode compared with hydrogen oxidation reaction at the anode, ORR is a major factor for a large drop of the efficiency of fuel cell. Up to date, expensive Pt has been used primarily as an efficient ORR catalyst.
The research team realized a record high activity by employing their catalyst as the cathode catalyst of alkaline membrane fuel cell (one type of hydrogen fuel cell). The team also demonstrated very high performance in proton exchange membrane fuel cell (PEMFC), in which the developed catalyst showed the activity of 320 A cm-3, exceeding 2020 US Department of Energy (DOE) activity target for non-precious metal catalyst (300 A cm-3).
"Our novel strategy for high-performance catalyst is expected to hasten the commercialization of hydrogen fuel cell, and the catalyst design can be also applied to other energy storage and conversion devices." says Prof. Joo.
More information: Young Jin Sa et al, A General Approach to Preferential Formation of Active Fe–NSites in Fe–N/C Electrocatalysts for Efficient Oxygen Reduction Reaction, Journal of the American Chemical Society (2016). DOI: 10.1021/jacs.6b09470
Journal information: Journal of the American Chemical Society