This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Team demonstrates halogen bonding for selective electrochemical separation, a path to sustainable chemical processing
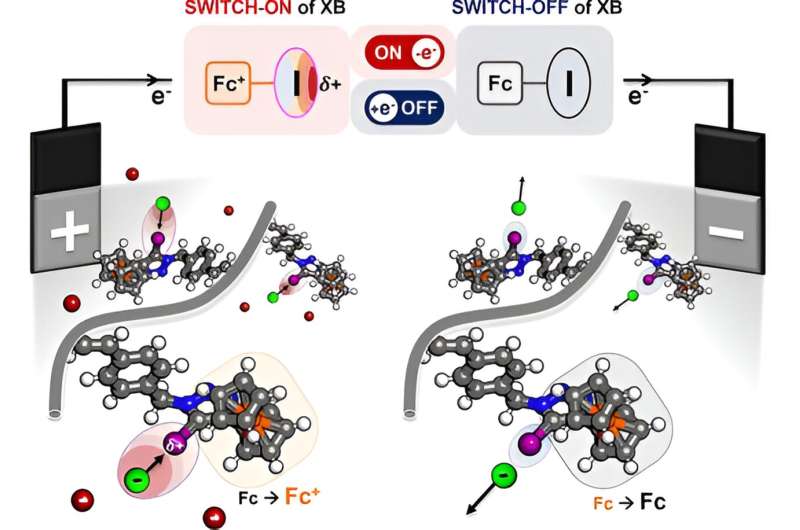
With a new polymer that only attracts certain substances from solutions when electrically activated, researchers have taken a major step towards sustainable chemical separation.
A team based at the University of Illinois Urbana-Champaign has reported the first demonstration of selective electrochemical separation driven by halogen bonding in the journal JACS Au.
They achieved this by engineering a polymer that modulates the charge density on a halogen atom when electricity is applied. The polymer then attracts only certain targets—such as halides, oxyanions, and even organic molecules—from organic solutions, a feature that has important implications for pharmaceuticals and chemical synthesis processes.
"Chemical separation is like making a sponge that only absorbs the chemical that you want from a mixture," said Xiao Su, a professor of chemical & biomolecular engineering and the project lead. "While electrochemical separation is used in some contexts, it can be quite challenging to ensure that they only soak up what is needed. In this work, we have made an 'electric sponge' at the molecular level that only picks out particular components of mixtures."
In industrial settings, chemical separation is often achieved through heat-based processes or membrane filtration, but these methods create material waste. Alternatives based on electrochemical mechanisms would minimize waste and benefit from sustainable sources of electricity. While such mechanisms are already used in applications like desalination, they are indiscriminate in the substances they attract.
The researchers achieved selective electrical separation with a chemical interaction called halogen bonding, in which a target molecule is attracted to a redox-responsive halogen donor polymer by the strong partial positive charge on halogen atom, called the "sigma hole."
The team exploited this interaction by engineering a polymer containing a halogen iodine atom and ferrocene, an active redox center that modulates the iodine's bonding strength when external electricity is applied. The iodine sigma hole is switched on when the ferrocene oxidizes, creating a strong positive charge that attracts negatively charged ions.
"Halogen bonding is a well-studied—if niche—area of fundamental chemistry, but our team is the first to take the concept and use it to develop a working 'sponge,'" said Nayeong Kim, a graduate student in Su's research group and the study's lead author. "The strength of halogen bonding is what enables selectivity, since it picks out ions that have high affinity to the halogen atom."
Su's research group designed the redox-active polymer then tested it in various organic solutions. After finding that the polymer could indeed select specific ions from a mixture, the presence of halogen bonding was confirmed using nuclear magnetic resonance and Raman scattering experiments. Su's group collaborated with chemical & biomolecular engineering professor Alex Mironenko, who led computational investigations of the polymer to understand the underlying mechanisms in the redox center's activation.
"Now that we have demonstrated molecular electrochemical separation, the next steps will involve refining and scaling the process," Su said. "That includes exploring scaling-up strategies, such as the cascade model, to enhance the purity of the final product, designing a continuous electrosorption system and then studying the process outside laboratory conditions."
Vijaya S. Jeyaraj, Johannes Elbert and Sung Jin Seo also contributed to this work.
More information: Nayeong Kim et al, Redox-Responsive Halogen Bonding as a Highly Selective Interaction for Electrochemical Separations, JACS Au (2024). DOI: 10.1021/jacsau.4c00265
Journal information: JACS Au