This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Modified solvents achieve vastly increased potentials for oxidation
A team of scientists headed by Professor Ingo Krossing, Professor of Molecular and Coordination Chemistry at the University of Freiburg's Institute of Inorganic and Analytical Chemistry, has succeeded in significantly increasing the potential for oxidation of and positive ions.
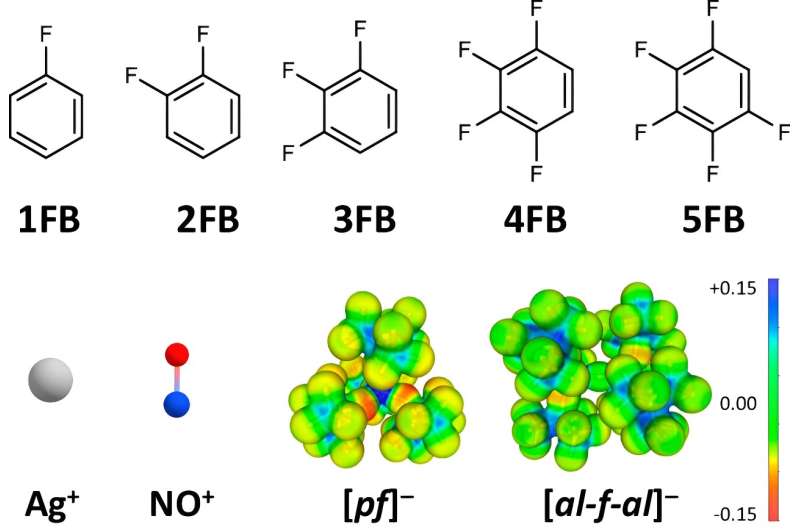
While the potential of these positive ions in conventional solvents and anions is up to +0.65 / +1.0 V vs. Fc+/0, the scientists demonstrated potentials of up to +1.50 / +1.52 V vs. Fc+/0. This was achieved with the use of particularly weakly interacting solvents and anions, with the work group focusing on strategic and polarity-maximizing substituted fluorinated benzene derivatives.
This new approach will in future enable redox reactions even with hard-to-oxidize systems or entirely new applications in the field of electrocatalysis or redox shuttles/mediators. The team's results have been published in Nature Communications.
The weaker the interaction with the positive ion, the stronger the potential for oxidation
Ag+ and NO+ positive ions are oxidizing agents widely used in chemistry and materials research. With the right conditions, they can selectively remove electrons from substrates.
Since these positive ions are very small and have a high charge density, they interact strongly with their environment. And it is this strong interaction with the environment, for example the anion or the solvent, that leads to the potential for oxidation of these positive ions being massively reduced.
In order to maximize the oxidation power of the dissolved positive ions, the scientists used particularly weakly coordinating anions (WCA) and solvents.
As solvents, the work group resorted to fluorinated benzene derivatives. To understand the properties of this molecule class, Dr. Johannes Hunger from the Max Planck Institute for Polymer Research assisted the research and determined the extremely important values of the dielectric constants as a solvent property.
This revealed that, in particular, the two-fold to four-fold fluorinated aromatic compounds in this aspect displayed higher values than conventional solvents such as dichloromethane or acetone.
While benzene itself or a single fluorinated benzene still interacts strongly with the Ag+ and NO+ positive ions, the interaction with all other fluorine atoms reduces.
"Besides the electrochemical measurements, we have determined the solid-state structures of compounds of the solvents and the positive ions using single-crystal X-ray diffraction and were able to show the interaction reduces the greater the degree of fluorination," explains co-author Dr. Malte Sellin.
"These almost undisturbed particles and their high potential for oxidation allow previously unachievable reactions," says Krossing.
"This enables a large number of new elementary chemical studies and potentially also entirely new applications. In the future, we'll understand even better how molecules behave in an oxidized state—simply because we can now also produce and study them."
More information: Christian Armbruster et al, Pushing redox potentials to highly positive values using inert fluorobenzenes and weakly coordinating anions, Nature Communications (2024). DOI: 10.1038/s41467-024-50669-3
Journal information: Nature Communications
Provided by University of Freiburg