This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers study effects of solvation and ion valency on metallopolymers

In a new paper published in JACS Au, researchers at the University of Illinois Urbana-Champaign analyzed the effects of solvation and ion valency on metallopolymers, with implications for critical materials recovery and recycling, and environmental remediation.
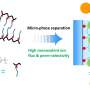
Chemical and biomolecular engineering (ChBE) professor Xiao Su led the research, which explored the science behind the selectivity "preferences" of monovalent and divalent anions towards redox polymers. In other words, why—when electrodes are coated with redox polymer films and potential is applied—one ion prefers the redox polymer while another does not.
"The idea is simple," Su said. "When you apply potential you bind the ion, and then you want to have a surface that gives you selectivity towards the ion that you want. Then, by applying the opposite potential, you can regenerate it. So you have a fully electrochemically-driven, green way of doing ion separations. Core to this process is understanding why ions prefer the electrode the way they do."
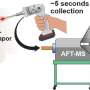
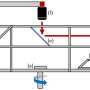
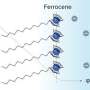
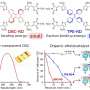
The team hypothesized that solvation plays a role in determining selectivity. Working with Jim Browning, Hanyu Wang and Mat Doucet at Oak Ridge National Laboratory, the team utilized neutron reflectometry (NR) to observe the swelling of films as well as the amount and distribution of water entering the polymer when potential was applied. In this case, they employed two thin redox-active metallopolymer films with different hydrophilic/hydrophobic characteristics—poly(vinyl ferrocene) (PVFc) and poly(3-ferrocenylpropyl methacrylamide) (PFPMAm)—and targeted the separation of rhenium from molybdenum.
A sequence of reducing/oxidative potential steps was applied to the PVFc and PFPMAm films in a solution containing rhenium and a comparable solution containing molybdenum—enough applied potential to respectively reduce or oxidize the films. They tracked the swelling using NR and spectroscopic ellipsometry (SE), and used electrochemical quartz crystal microbalance (EQCM) to monitor net mass change at the interface. Collaborators at the Pacific Northwest National Laboratory, Manh Nguyen and Vanda Glezakou, carried out ab initio molecular dynamics (AIMD) calculations—a powerful tool that simulates the physics happening at the electrode.
The NR, SE, and EQCM were employed in situ, which gave the researchers a unique opportunity to gain a clearer molecular picture of the behaviors than ever before.
"Neutrons were key to track the movement of water in the polymers under real working conditions," said Riccardo Candeago, the ChBE Ph.D. student who is the first author on the paper. "By using multiple in situ techniques as well as simulations we got a full picture of our system."
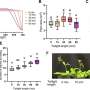
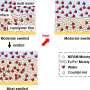
Their analysis showed that the PVFc and PFPMAm films both swell in the presence of rhenium, a monovalent anion, but not molybdenum, a divalent anion.
"We found that solvation does indeed play a role: PVFc, the more hydrophobic polymer, prefers the least solvated anion—in this case rhenium," Su said. "And the divalent anions, when they come in, they actually tend to electrostatically cross-link the film so it's not as regenerable. Basically, these films are very good at capturing these single charge ions."
Su said that their findings will guide the development of better systems that involve ion separations such as materials recycling and metal recovery. For example, rhenium is both a valuable metal used as a catalyst and an analog for technetium, a radioactive element which is hard to separate from nuclear waste, making rhenium capture of great importance to strategic metals recycling. But these advanced characterization methods can also be used for broader classes of polymers, not just metallopolymers, meaning better systems for processes like water treatment and environmental remediation.
"This understanding was only possible by using these tools, and it can give us a lot of insight," Su said. "So when we're designing systems that can capture ions with different charges as well as ions with different solvation properties, it can help us establish some design principles. Overall, it's a very fundamental study, but is one that has practical applications down the line."
The paper "Unraveling the Role of Solvation and Ion Valency on Redox-Mediated Electrosorption through In Situ Neutron Reflectometry and Ab Initio Molecular Dynamics" is available online.
More information: Riccardo Candeago et al, Unraveling the Role of Solvation and Ion Valency on Redox-Mediated Electrosorption through In Situ Neutron Reflectometry and Ab Initio Molecular Dynamics, JACS Au (2024). DOI: 10.1021/jacsau.3c00705
Journal information: JACS Au