This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study presents new pathway for electrochemically controlling ion selectivity
A new study by researchers at the University of Illinois Urbana-Champaign advances fundamental knowledge about the role of solvation in ion binding and presents a new pathway for electrochemically controlling ion selectivity. The study was published in JACS Au.
The team, led by Chemical & Biomolecular Engineering professor Xiao Su and recently graduated Ph.D. student Raylin Chen, is building on their prior work exploring electrochemical separations of ions, which has revealed that a critical mechanism for binding ions is solvation.
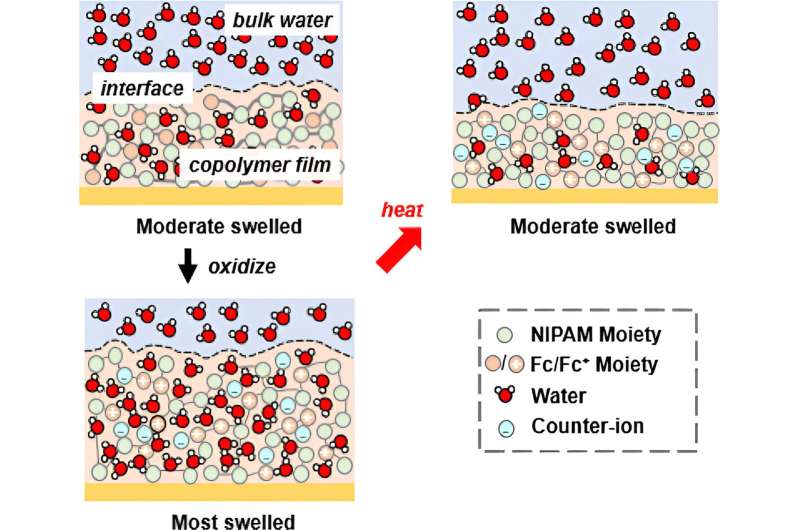
Here, the researchers set out to control the solvation of a polymer and use that to bind different ions specifically to do it via an electrochemical process through a unique approach. To accomplish that, they created a copolymer system containing N-isopropyl acrylamide (NIPAM)—which has previously been shown to be a temperature-responsive material—and introduced redox-active units to it.
Because the copolymer has two units—one that is electroactive and one that is the original thermo-responsive unit—there are now two pathways available to control solvation.
"By tuning potential, we basically force NIPAM to take water or release water based on electrochemistry," Su said. "So instead of doing a thermal transition on NIPAM, we're doing an electrochemical transition on NIPAM."
The copolymer yielded gel films, which became their platform for solvation-controlled ion separations. The researchers were able to run tests using in situ ellipsometry, a method they created that allows them to observe the thickness of the film swelling and deswelling in response to addition or release of water based on electrochemistry.
Collaborating with a team at Oak Ridge National Laboratory led by Jim Browning, Hanyu Wang, and Mat Doucet, they employed an advanced technique called neutron reflectometry (NR).
"Basically, neutrons allow you to see things that you normally cannot see using standard techniques like X-rays," Su said. "Neutrons are very sensitive to water, so neutrons can tell you how much water there is in the content of a material."
NR allowed them to see solvation, or how much water is distributed across the film, and showed that under potential, the film swells and takes in water. Su said that the researchers were able to demonstrate that with different degrees of water uptake, they could control ion selectivity.
Because their work offers a system that can be activated both by temperature and by electrochemical potential, it sets the stage for a materials platform that can be used in the future under different sustainable scenarios—for example, powered by renewable energy or by waste heat.
"Our work advances the area of electrochemical separations for water treatment and resource recovery by providing more in-depth understanding into the molecular mechanisms," Su said. "To develop technologies that are more energy efficient and selective, it is important to exert more precise control over ion binding mechanisms. We hope our work contributes towards this goal by elucidating the importance of solvation."
More information: Raylin Chen et al, Thermo-Electro-Responsive Redox-Copolymers for Amplified Solvation, Morphological Control, and Tunable Ion Interactions, JACS Au (2023). DOI: 10.1021/jacsau.3c00486