November 9, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Atom-by-atom solvation recorded for the first time
A team of chemists and physicists at Aarhus University, in Denmark, working with a colleague from Universitat de Barcelona, in Spain, has recorded atom-by-atom solvation for the first time. In their study, published in the journal Nature, the group designed a process to manipulate sodium and xenon atoms with a droplet of helium at very cold temperatures to capture what they describe as snapshots of the solvation process over time. Combined, these produce a movie depicting the action. A Research Briefing on the work has been published in the same journal issue.
Solvation is the dissolving of a solute in a solvent—when salt dissolves in water, for example. The action does not stop just because the solute has dissolved; instead, the solvents continue to interact with the material that has been dissolved.
Prior research has shown that such interactions can be quite complicated, which is why chemists want to know more about what happens. One way to find out would be to film the action and play it like a movie. This simple concept has proven to be exceptionally difficult, however—so difficult that it was not accomplished until recently by the team in Denmark.
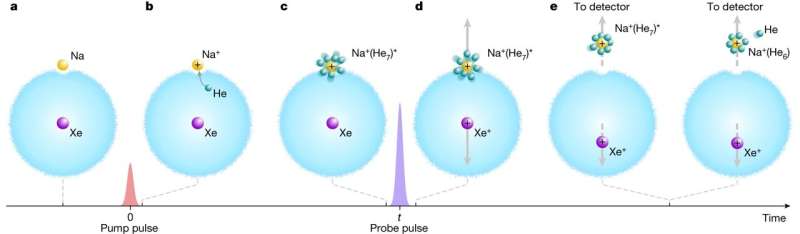
To achieve their feat, the researchers began by trapping a single xenon atom inside a droplet of liquid helium that had been cooled to -255°C and then adding a single sodium atom to the outer edge of the drop. They fired a short pulse from a laser at the sodium atom to convert it into a positively charged ion, setting off solvation—the helium atoms began to stick to the sodium ion.
The team then fired another laser pulse, this time at the xenon atom, changing it to a positively charged ion. The two ions repelled one another to such an extent that the sodium ion, with its attached helium atoms, was pushed out of the droplet and onto a detector, which allowed for the capture of a snapshot of what was occurring.
The researchers then repeated the process, each time waiting longer to fire the second pulse. They were able to create what they describe as progressive snapshots of the action. Then, once they had multiple sequential snapshots, they stitched them together to create a movie that depicted the solvation process in action.
More information: Simon H. Albrechtsen et al, Observing the primary steps of ion solvation in helium droplets, Nature (2023). DOI: 10.1038/s41586-023-06593-5
Helium droplets help to visualize the start of ion solvation, Nature (2023). DOI: 10.1038/d41586-023-02950-6
Journal information: Nature
© 2023 Science X Network