March 22, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists synthesize and characterize novel helically chiral oxonium ion with a stereogenic oxygen atom center
A team of chemists at the University of Oxford, working with a colleague from Colorado State University, has synthesized and characterized a novel, helically chiral oxonium ion with a stereogenic oxygen atom center for the first time. In their research, reported in the journal Nature, the group studied oxonium ions to come up with a way to create a pentacyclic oxonium salt with a chiral center.
Chiral organic molecules generally have an optical center based on a carbon atom, though some work has been done with the use of trivalent nitrogen. Prior researchers have looked at the possibility of using an oxygen atom, but the means for doing so was believed to be particularly difficult—thus, it has not been pursued. In this new effort, the research team found after studying oxonium ions that triaryl oxonium ions might be used to create a stable chiral compound with an oxygen atom at its center because they tend to be stable, isolable, and crystalline.
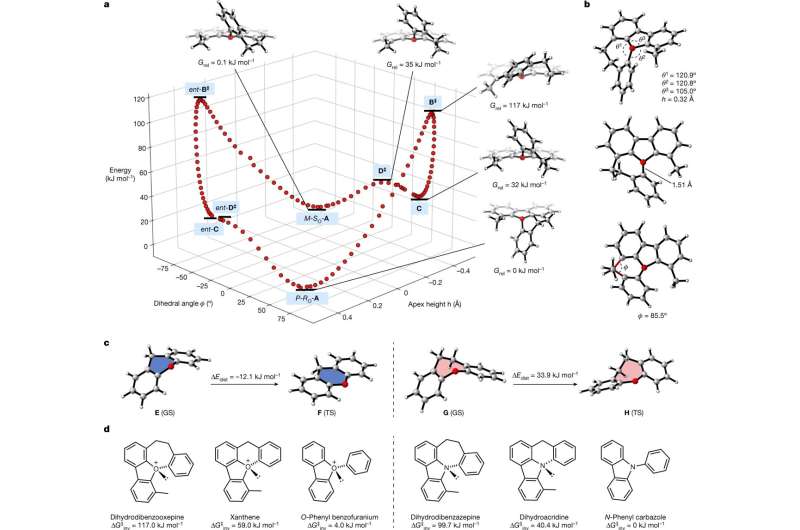
The team began their effort by noting that such a compound might be created if it were embedded in a suitable, flexible ring system as it would slow down inversion. They then began investigating suitable candidates using quantum mechanical theories. So they used an oxygen atom that was positively charged as the center atom. The oxonium ion was obtained by protonation of a pyridine-2-yl-substituted 1,4-benzodioxane derivative using triflic acid. The research team then added a trio of aromatic substituents tied together using seven-atom and five-atom rings. The result was a three-dimensional compound with slow racemization.
The team then studied the inversion rate of the compound using NMR spectroscopy along with liquid chromatography. They also used X-ray diffraction to analyze its configuration. They found the seven-ring version had a half life of more than a month when maintained at room temperature.
The work demonstrates that chiral organic molecules with oxygen atoms at their center can be synthesized. It also suggests that they might possibly be used as catalysts or as an ingredient in the production of various types of medicines.
More information: Owen Smith et al, Control of stereogenic oxygen in a helically chiral oxonium ion, Nature (2023). DOI: 10.1038/s41586-023-05719-z
Journal information: Nature
© 2023 Science X Network