April 9, 2018 report
Reaction adds chiral groups to nitrogen rings without first installing reactive groups

A trio of researchers at the University of Cambridge has come up with a new way to control adding chirality to nitrogen rings without first installing reactive groups on them. In their paper published in the journal Science, Robert Phipps and grad students Rupert Proctor and Holly Davis, describe the new reaction process and possible uses for it.
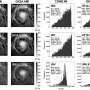
As the researchers note, nitrogen rings with associated chiral groups (groups that are asymmetric in nature) are common in agricultural, pharmaceutical and bioactive products, and chemists would like to have methods of adding groups with certain kinds of chirality to nitrogen to a cyclic compound in which one of the rings is not a carbon atom. Prior efforts to do so have involved catalytic asymmetric reduction reactions, but they have suffered from the need to pre-install reactive groups and require many complicated steps. Others have tried using a Minisci-type reaction, but thus far, it has resulted in the addition of other groups to the rings. In this new effort, the researchers also employ a Minisci-type reaction, though in a different way—the result is a process that has fewer steps, does not require pre-installing reactive groups and allows for much tighter control over ring positioning.
The reaction in the new method involves two catalysts that add certain radicals to a nitrogen heterocyclic compound derived from an arene (a heteroarene)—the result is a chiral core on a carbon atom that is itself attached to a ring. To get the reaction going, the researchers aimed a beam of light from a blue LED at the mixture causing an acid in the chiral catalyst to activate the heterocycle.
The researchers suggest the reaction technique could prove especially useful for developing medicines because it would be very easy to scale for manufacturing purposes. They note that their work very likely has commercial applications, but have at this time chosen not to pursue such options. Instead, they hope their work will be adopted and used by other researchers working on other pressing chemistry problems, particularly those involved in developing medications.
More information: Rupert S. J. Proctor et al. Catalytic enantioselective Minisci-type addition to heteroarenes, Science (2018). DOI: 10.1126/science.aar6376
Abstract
Basic heteroarenes are a ubiquitous feature of pharmaceuticals and bioactive molecules, and Minisci-type additions of radical nucleophiles are a leading method for their elaboration. Despite many Minisci-type protocols that result in the formation of stereocenters, exerting control over the absolute stereochemistry at these centers remains an unmet challenge. We report a process for addition of prochiral radicals, generated from amino acid derivatives, to pyridines and quinolines with excellent control of both enantioselectivity and regioselectivity. An enantiopure chiral Brønsted acid catalyst serves both to activate the substrate and induce asymmetry, while an iridium photocatalyst mediates the required electron transfer processes. We anticipate that this method will expedite access to enantioenriched small-molecule building blocks bearing versatile basic heterocycles.
Journal information: Science
© 2018 Phys.org