Nitrogen-15 isotope can trigger asymmetric autocatalytic reactions toward chiral organic compounds
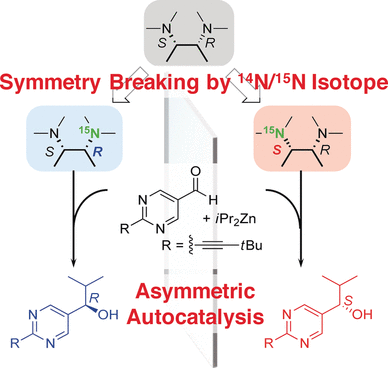
The preparation of chiral compounds as intermediates in drug synthesis is one of the most important targets in synthetic organic chemistry. Japanese scientists have now shown that the autocatalytic preparation of a chiral intermediate can be triggered by a compound bearing hidden chirality, which consisted of nothing more than the difference between the isotopes nitrogen-15 and nitrogen-14. The study is published in the journal Angewandte Chemie.
Nitrogen-15 is one of the two naturally occurring nitrogen isotopes—although with an abundancy of only 0.37% compared to that of 99.63% for nitrogen-14. However, nitrogen-15 is frequently employed as a tracer isotope in biology, and it is a source of the radioisotope carbon-15 that is used in nuclear medicine imaging applications. A team of scientists led by Kenso Soai at Tokyo University of Science, Japan, have found a rather different application: as an initiator for the asymmetric autocatalytic reaction to prepare a chiral organic intermediate, that is, a compound distinguishable from its mirror-image.
Autocatalytic reactions are attractive in synthesis because once a product is formed it serves as a catalyst for the propagation of the reaction, making the use of external catalysts obsolete. This is especially attractive for asymmetric catalysis which often employs expensive and sophisticated compounds as catalysts. In asymmetric autocatalysis, a chiral trigger initiates the reaction. Soai and his team were particularly interested in a nitrogen-group-containing trigger molecule that relies on the smallest chiral induction possible: the difference of atomic mass between nitrogen-14 and nitrogen-15 is only 7%.
The difference is so small that the scientists could not detect it by conventional chiral analysis. But its effect could be shown as it induced asymmetry in the reaction of a pyrimidine moiety, which is a common building block in many biological molecules, and an alkylation reagent. The resulting pyrimidine alcohol was clearly chiral with one mirror-image dominating the other. "This result is the first example of enantioselective induction by chirality using only the nitrogen isotope 14N/15N difference," the scientists reported. And it is a remarkable example of the effects that hidden chirality can have in synthesis.
More information: Arimasa Matsumoto et al. Asymmetric Induction by a Nitrogen 14N/15N Isotopomer in Conjunction with Asymmetric Autocatalysis, Angewandte Chemie International Edition (2016). DOI: 10.1002/anie.201608955
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley




















