Artificial enzyme for asymmetric synthesis using a synthetic chiral polymer
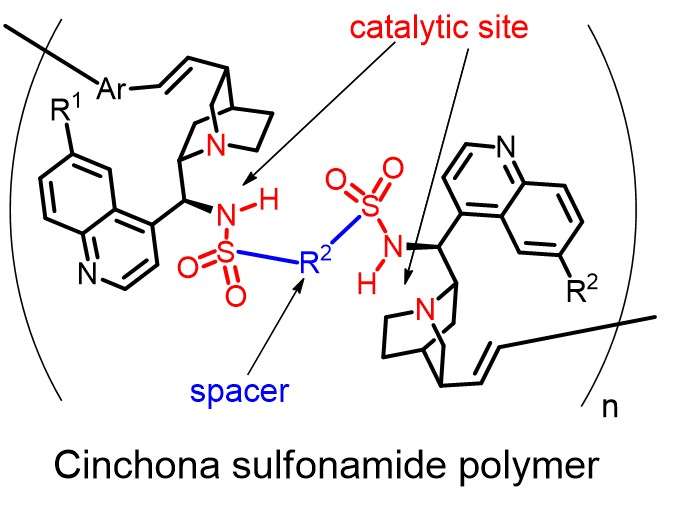
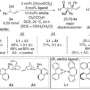
Enzymes, high-molecular-weight chiral polymeric compounds, are complex biological catalysts. Capture of the substrate molecule, catalyzing the reaction, and release of the product are three important events performed by enzymes. In order to accomplish these important events using a synthetic catalyst, it must necessarily have a large molecular weight. However, synthetic chiral polymers for this purpose have not been designed so far. A research team in the Department of Environmental and Life Science at Toyohashi University of Technology has investigated a novel synthetic method for preparing chiral polymers containing repeating units of cinchona sulfonamide.
Lead author Shohei Takata said, "After testing many reaction conditions for the polymerization, we have synthesized chiral polymers containing cinchona sulfonamide repeating units. Chiral polymers are easily prepared by the method we established."
"We have found that Mizoroki-Heck coupling was successful in synthesizing cinchona sulfonamide polymers," explains project leader Shinichi Itsuno. "Moreover, our chiral polymers showed high catalytic activity in asymmetric reactions." Various kinds of such chiral polymers may be synthesized using this newly developed methodology to obtain various types of synthetic enzymes for specific reactions.
Furthermore, the chiral polymers developed in this study are insoluble in the usual organic solvents or water. The insoluble polymeric catalysts can be packed into a column, into which the substrate compounds can be introduced. The desired product can then be continuously obtained from the column. Without a usual reaction vessel, a continuous flow system may be possible using the polymeric catalyst. The flow system is a necessary technology for the automation of fine chemical syntheses.
More information: Shohei Takata et al, Synthesis of cinchona alkaloid sulfonamide polymers as sustainable catalysts for the enantioselective desymmetrization of cyclic anhydrides, RSC Adv. (2016). DOI: 10.1039/C6RA14535C
Provided by Toyohashi University of Technology