November 24, 2020 feature
Regulating the reactivity of black phosphorous through protective chemistry
Chemists can rationally regulate the reactivity of molecules and functional groups in both industrial and laboratory-based synthetic organic chemistry processes. The concept can be applied to inorganic nanomaterials including two-dimensional (2-D) black phosphorus (BP) nanosheets. For example, scientists can "shut down" the high reactivity of few layer or monolayer black phosphorus, when the compound is not in use, to resume its activity upon application. In a new report now published on Science Advances, Xiao Liu and a team of scientists in physics, biomaterials, chemical engineering and biological chemistry in China developed a protective chemistry-based method to regulate the reactivity of black phosphorus.
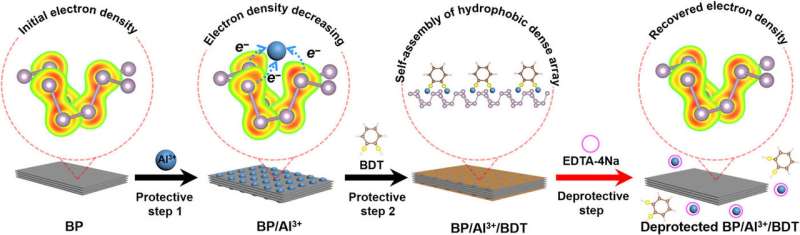
To initiate the protective step, they bound aluminum cations (Al3+) with lone pairs of electrons from black phosphorous (BP) and decreased the electron density on the black phosphorus surface. They completed the process with an oxygen/water-resistant layer via the self-assembly of hydrophobic (water-hating) 1,2-benzenedithiol (BDT) on the black phosphorus/aluminum (BP/Al3+) conjugate. The protective step produced a stable compound with low reactivity. By using a chelator treatment process, Liu et al. subsequently achieved deprotection of the BP/Al3+/BDT complex to remove aluminum cations and BDT from the black phosphorus surface. In this way, they recovered the electron density of black phosphorus using the de-protective process to restore reactivity to the compound.
Tuning the properties of nanomaterials
Liu et al. rationally developed a protective chemistry-based method to control the reactivity of black phosphorus during this work. In nanoscience, researchers can precisely tune the properties of nanomaterials to obtain desired characteristics. Regulating the reactivity of nanomaterials is critical for multistep programmable applications. Some nanomaterials can be protected to reduce their reactivity under specific conditions and restore activity after successful deprotection. Researchers have therefore proposed a range of selective and efficient strategies to regulate the reactivity of functional groups in organic chemistry.
However, the existing well-established organic protective-deprotective processes cannot be applied to inorganic nanomaterials due to a lack of similar functional groups. As a result, an efficient and simple approach to control the reactivity of inorganic materials remains to be developed. To accomplish this, the team began binding black phosphorus with aluminum cations (Al3+) to decrease the surface electron density and effectively decrease its reactivity. The protective process produced an arrangement of the hydrophobic (water-hating) 1,2-benezendithiol (BDT) molecule in the presence of black phosphorus and Al3+ cations to offer an ultra-stable complex (BP/ Al3+ /BDT). The compound was stable under ambient conditions for up to two months without alterations. The team can deprotect the unstable complex through chelator treatment.
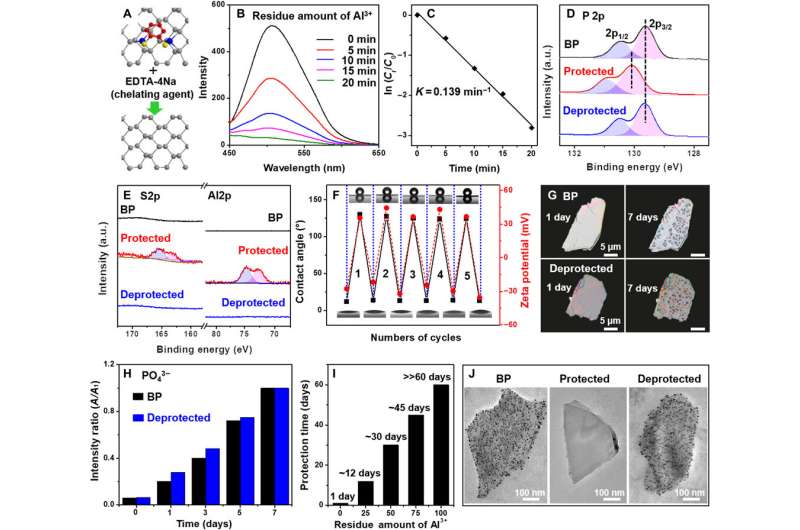
Synthesis and characterization of the BP/Al3+/BDT compound
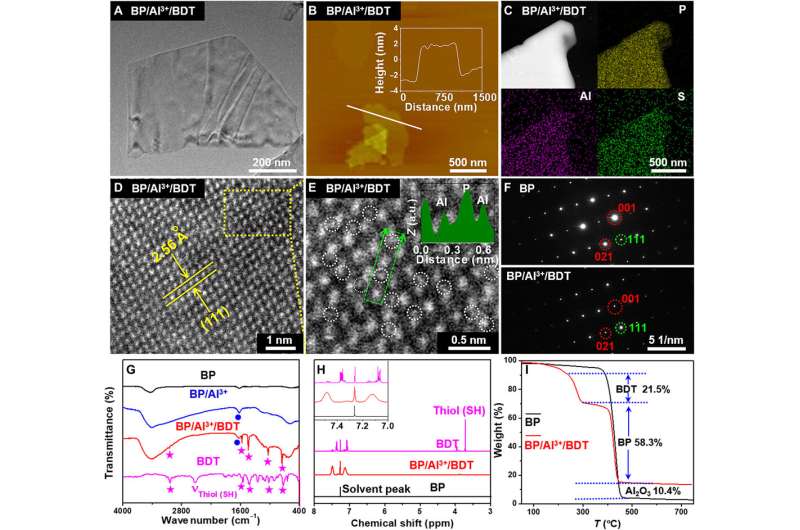
Liu et al. synthesized and characterized (tested) the bulk black phosphorous (BP) by following a previously developed method. The team first obtained BP nanosheets by sonicating the powdered form of the molecule, then using scanning electron microscopy and transmission electron microscopy (TEM), they observed the size of BP. High resolution TEM too provided insight to the nanosheet structure and atomic force microscopy revealed the thickness of BP with four to six individual layers of phosphorene layers. Using X-ray diffraction and Raman spectra, the team determined the crystal characteristics of BP to be similar to its bulk form. At the protective step of cationic binding to the BP surface, Liu et al. mixed BP with aluminum chloride (AlCl3) in an ethanol solution. They then characterized the successful attachment of Al3+ cations to the BP surface to strengthen the protection of BP by employing X-ray photoelectron spectroscopy. The team observed the nano-morphology of the compound and studied the surface conformation using scanning transmission electron microscopy (STEM) and further verified its structure with Fourier transform infrared (FTIR) spectroscopy and proton nuclear magnetic resonance (1HNMR).

Regulating the reactivity of the BP/Al3+ /BDT compound
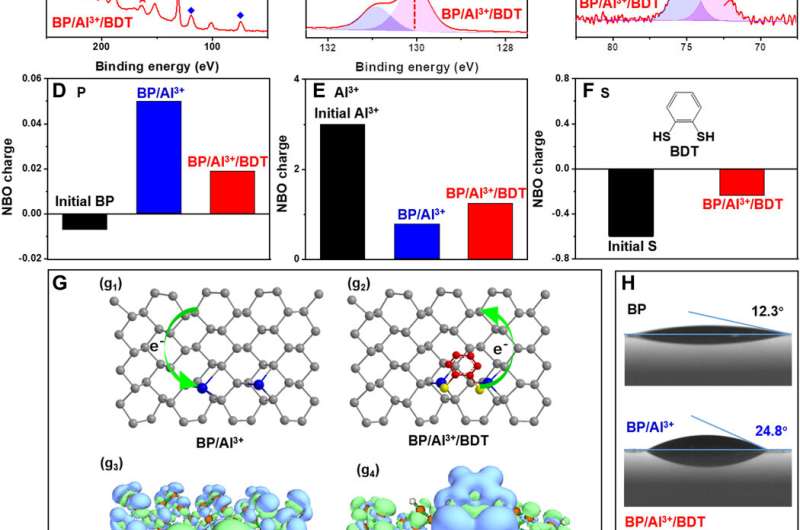
Liu et al. investigated the reactivity of the BP/Al3+/BDT complex by employing a variety of optical techniques in materials science to understand its structure. The corresponding images showed the evolution of the crystal structure and long-term stability under ambient conditions. The stability of the BP/Al3+/BDT complex was superior to black phosphorus alone and the results highlighted the practicality of embedding black phosphorus in the compound. The team credited the reduced reactivity of the complex structure to two factors; first, the aluminum cation binding the black phosphorus surface rendered lower chemical reactivity. Second, the self-assembled hydrophobic dense array on the surface of black phosphorus isolated the molecule from oxygen and water to prevent further degradation, improving the stability of the compound.
Deprotecting the BP/Al3+/BDT compound
The research team deprotected the ultra-stable complex by removing the aluminum cation from the surface of the compound. They accomplished this step with a conventional metal ion chelator—edetate sodium (EDTA-4Na). During the process, Liu et al. also removed the hydrophobic BDT molecules together with aluminum cations, to obtain the resulting hydrophilic (water-loving) surface with negative zeta potential, similar in form to the original black phosphorus molecule. The protective-deprotective regulation process allowed the scientists to reversibly control the reactivity of black phosphorus. The results suggest the ability to efficiently regulate the reactivity of the molecule in practice.
Outlook
In this way, Xiao Liu, and colleagues developed a new protective chemistry-based approach to rationally regulate the reactivity of black phosphorus (BP). During the protective step, they bound aluminum cations (Al3+) to the lone pair of electrons on BP to decrease its surface electron density. This protective step enhanced the functionalization of the hydrophobic (water-hating) 1,2-benezendithiol (BDT), on the BP/ Al3+ conjugate surface to form a dense hydrophobic layer, which greatly reduced the reactivity of black phosphorus. The team then used a chelator to remove the aluminum cations from black phosphorus to revert the molecule back to its original high electron density, hydrophilic (water-loving) surface. The deprotected BP showed high reactivity in its original state. The method provided a tunable approach to manipulate the reactivity of BP, which is hard to achieve through conventional functionalization. This protective strategy can be explored to regulate the reactivity of nanomaterials to create futuristic programmable nanostructures for applications in materials science.
More information: Liu X. et al. Regulating the reactivity of black phosphorus via protective chemistry, Science Advances, 10.1126/sciadv.abb4359
Sperling R. A. & Parak W. J., Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philosophical Transactions of the Royal Society A, doi.org/10.1098/rsta.2009.0273
Li L. et al. Black phosphorus field-effect transistors. Nature Nanotechnology, doi.org/10.1038/nnano.2014.35
Journal information: Science Advances , Philosophical Transactions of the Royal Society A , Nature Nanotechnology
© 2020 Science X Network