A novel approach of improving battery performance
New technological developments by UNIST researchers promise to significantly boost the performance of lithium metal batteries in promising research for the next-generation of rechargeable batteries. The study also validates the principle of enhanced battery performance via the real-time in situ observation of charge-discharge cycling.
This breakthrough has been led by Professor Hyun-Wook Lee in the School of Energy and Chemical Engineering at UNIST in collaboration with the Agency for Science, Technology and Research (A*Star) in Singapore.
Lithium metal batteries are a type of rechargeable battery that has lithium as an anode. Among a number of different cathode materials, lithium metal has the lowest driving voltage and boasts about 10 times more capacity than conventional graphite anodes. Therefore, it has been gaining much attention as a potential next-generation anode material for electric vehicles and large-scale energy storage systems.
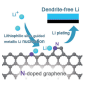
While lithium metal anodes represent an ideal candidate for high energy density batteries, their use as anodes in commercial cells requires more development. For example, lithium metal tends to grow into dendritic structures during the continuous charging/discharging processes of a battery, which may result in poor performance. This is because this dendritic structure on the lithium metal surface layer triggers internal short circuits by piercing through the battery separator.
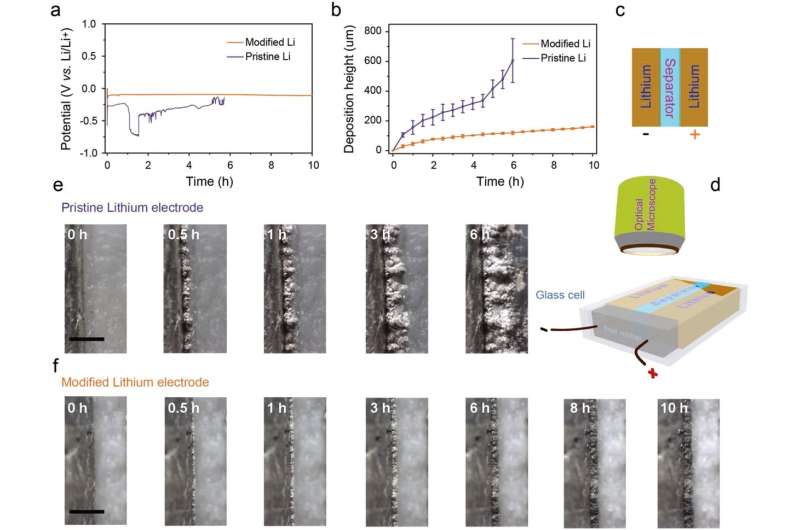
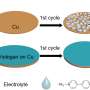
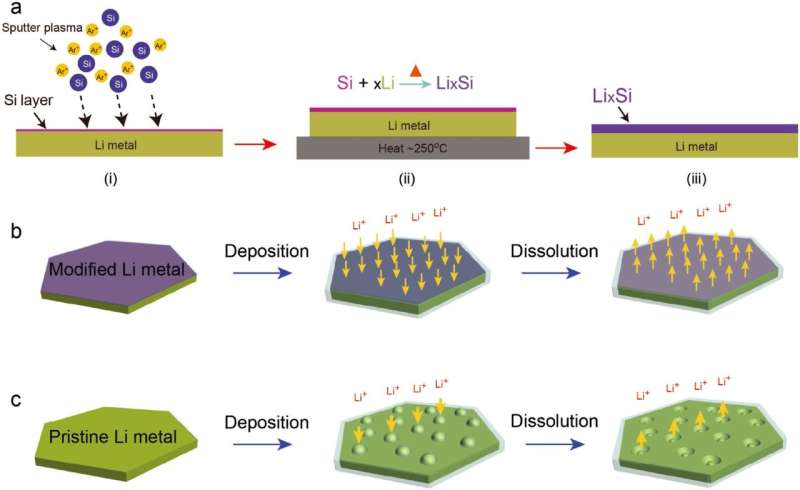
In the study, the research team suppressed dendritic growth by coating the lithium foil with a lithium silicide (LixSi) layer. Results showed excellent electrochemical performance in terms of rate capability and cycle stability.
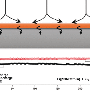
In situ optical microscopic observation was also carried out to monitor the electrochemical deposition of lithium on the LixSi‐modified lithium electrodes and the bare lithium electrode. It was observed that a much more uniform lithium dissolution/deposition on the LixSi-modified lithium anode can be achieved as compared to the bare lithium electrode.
"Our study provides direct observation of electrochemical behavior, volume expansion, as well as the lithium dendrite growth of lithium metal anodes," says Professor Lee. "Applying this in real batteries will also help contribute to the commercialization of lithium metal batteries."
This research has been published in Advanced Materials.
More information: Wei Tang et al. Lithium Silicide Surface Enrichment: A Solution to Lithium Metal Battery, Advanced Materials (2018). DOI: 10.1002/adma.201801745
Journal information: Advanced Materials