Reducing the footprint of a greenhouse gas more potent than carbon dioxide
USC scientists have unlocked a new, more efficient pathway for converting methane - a potent gas contributing to climate change - directly into basic chemicals for manufacturing plastics, agrochemicals and pharmaceuticals.
In research published on Dec. 4 in the Journal of the American Chemical Society, chemists at USC Loker Hydrocarbon Research Institute say they have found a way to help to utilize this abundant and dangerous greenhouse gas, which is generally burnt or flared to produce energy.
Among common greenhouse gases, carbon dioxide is often cited as the largest culprit for trapping heat on earth, contributing to climate change. However, it is not the most potent.
That distinction belongs to methane. According to the Intergovernmental Panel on Climate Change, methane traps heat and warms the planet 86 times more than carbon dioxide over a 20-year horizon.
More fuel, fewer emissions, reduced energy use
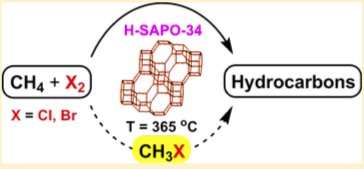
Lead author Patrice T. D. Batamack, senior author G. K. Surya Prakash and Thomas Mathew of the USC Loker Hydrocarbon Research Institute used a catalyst called H-SAPO-34, derived from a class of nanoporous crystals called zeolites.
This simple method of converting methane directly to ethylene and propylene, or olefins, would replace what are traditionally difficult, expensive, and inefficient processes that add greenhouse gases to the atmosphere. The majority of ethylene and propylene is produced from petroleum oil and shale liquid cracking, which consumes enormous amounts of energy.
When USC's first Nobel Prize winner, George Olah, converted methane to olefins in 1985, the process required three steps. Since then, researchers have reduced it to two steps, but the Loker team is the first to realize the conversion with a single catalyst based on zeolites.
"Contact time is the key for this effective and simple catalyst to produce usable fuel from methane. In real estate, they say, location, location, location. In chemistry, it is all about condition, condition, condition," said Prakash.
Global methane emissions have surged since 2007 and output is particularly bad in the United States. According to a recent Harvard University study, the United States could be solely responsible for as much as 60 percent of the global growth in human-caused atmospheric methane emissions during this century.
Contributing to the global surge is the increased supply of livestock and rice fields in countries like India and China, the two leaders in total methane output, according to the World Bank.
'If carbon is the problem, carbon has to be the solution'
While being the most potent of our popular greenhouse gases, and even after the largest methane leak in U.S. history at the Aliso Canyon natural gas storage facility a few years ago, there are no signs that methane's abundant production will slow down anytime soon.
Shale fracking and other resource extraction techniques are increasing natural gas reserves, and the Loker scientists believe methane may soon become the most popular of all raw materials for producing petrochemical products.
About 30 years ago, Prakash and his mentor Olah first began refining the concept of "The Methanol Economy," a host of methanol-based solutions mitigating the production cycle of the greenhouse gases that are accelerating climate change.
While similar in structure and name, methane is not directly interchangeable with methanol, although most methanol is synthetically produced from methane. Methane is a naturally occurring gas and the simplest one-carbon compound containing hydrocarbon.
By further reducing the steps necessary to efficiently convert methane to olefins, the scientists at Loker may have brought us that much closer to realizing one of the original steps laid out in "The Methanol Economy."
"If carbon is the problem, carbon has to be the solution. There is plenty of methane to go around in the world and it is become easier and safer to turn it into products that we can actually use,'" said Prakash.
More information: Patrice T. D. Batamack et al, One-Pot Conversion of Methane to Light Olefins or Higher Hydrocarbons through H-SAPO-34-Catalyzed in Situ Halogenation, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b10725
Journal information: Journal of the American Chemical Society
Provided by University of Southern California