November 30, 2017 report
A way to convert methane directly to methanol or acetic acid under mild conditions
(Phys.org)—A team of researchers with affiliations to Tufts University, Oak Ridge National Laboratory and Argonne National Laboratory has developed a way to convert methane directly to methanol or acetic acid under mild conditions. In their paper published in the journal Nature, the group describes the procedure and suggests their technique might lead to other efforts aimed at optimizing catalysts for the direct conversion of methane to useful liquid chemicals. Ive Hermans with the University of Wisconsin outlines current techniques for converting the gas to other products in a News & Views piece in the same journal issue, and also discusses the work done by the team in this new effort.
For some time, chemists have sought a catalyst that could be used to convert methane directly to other liquid chemicals under mild conditions, but have met with little success. In recent times, efforts have intensified, as Hermans notes, as the gas is more abundant due to an increase in fracking—methane is a main component of natural gas. In this new effort, the researchers report the development of just such a technique, though they acknowledge that it is not likely suitable for commercialization.
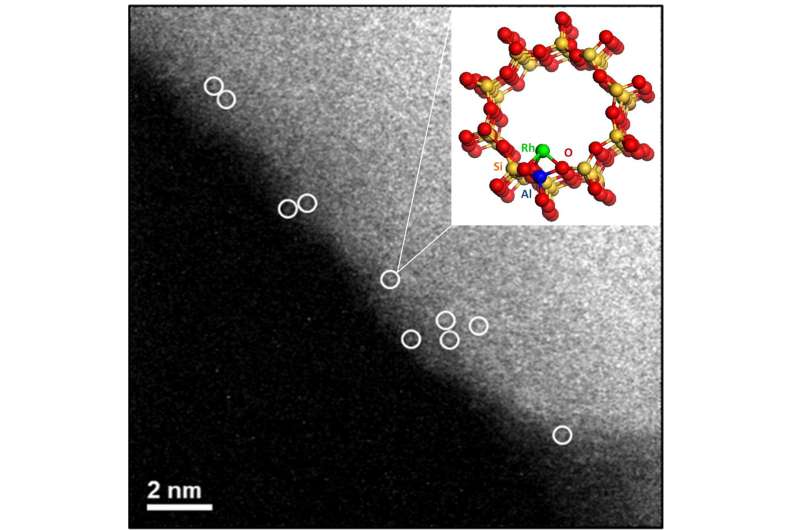
The new process developed by the researchers involves showing that a mononuclear rhodium species based on zeolite or titanium dioxide is able to support catalyzing the direct conversion of methane to methanol or acetic acid. Methanol, also called methyl alcohol or wood alcohol, is used for a variety of purposes. Acetic acid is a colorless liquid found in some foods and cooking ingredients such as vinegar. Their process involved using rhodium as a catalyst, dispersing it on either the zeolite or the titanium dioxide in the presence of oxygen and carbon monoxide. The end product was dependent on the acidity of the supporting materials.
The researchers report that the process resulted in the production of approximately 0.4 kilograms of acetic acid per kilogram per hour, which, they note, is likely too low for commercial purposes. But it is still a remarkable step forward, Hermans notes, adding that it likely will lay the groundwork for other studies aimed at making useful products from methane.
More information: Junjun Shan et al. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts, Nature (2017). DOI: 10.1038/nature24640
Abstract
An efficient and direct method of catalytic conversion of methane to liquid methanol and other oxygenates would be of considerable practical value. However, it remains an unsolved problem in catalysis, as typically it involves expensive or corrosive oxidants or reaction media5,6,7,8 that are not amenable to commercialization. Although methane can be directly converted to methanol using molecular oxygen under mild conditions in the gas phase, the process is either stoichiometric (and therefore requires a water extraction step) or is too slow and low-yielding to be practical. Methane could, in principle, also be transformed through direct oxidative carbonylation to acetic acid, which is commercially obtained through methane steam reforming, methanol synthesis, and subsequent methanol carbonylation on homogeneous catalysts. However, an effective catalyst for the direct carbonylation of methane to acetic acid, which might enable the economical small-scale utilization of natural gas that is currently flared or stranded, has not yet been reported. Here we show that mononuclear rhodium species, anchored on a zeolite or titanium dioxide support suspended in aqueous solution, catalyse the direct conversion of methane to methanol and acetic acid, using oxygen and carbon monoxide under mild conditions. We find that the two products form through independent pathways, which allows us to tune the conversion: three-hour-long batch-reactor tests conducted at 150 degrees Celsius, using either the zeolite-supported or the titanium-dioxide-supported catalyst, yield around 22,000 micromoles of acetic acid per gram of catalyst, or around 230 micromoles of methanol per gram of catalyst, respectively, with selectivities of 60–100 per cent. We anticipate that these unusually high activities, despite still being too low for commercial application, may guide the development of optimized catalysts and practical processes for the direct conversion of methane to methanol, acetic acid and other useful chemicals.
Journal information: Nature
© 2017 Phys.org