July 20, 2017 report
Converting carbon dioxide to methane using iron and sunlight
(Phys.org)—A combined team of researchers from Université Paris Diderot in France and Universidad Nacional de Córdoba in Argentina has discovered a reaction process that can be used to convert carbon dioxide to methane. In their paper published in the journal Nature, the team describes their technique, how well it worked, and their ideas for improving it.
As human endeavors continue to introduce carbon dioxide into the atmosphere, leading to global warming, scientists around the world seek alternative ways to reduce both the amount of the gas emitted into the air and the amount that is already there. In this new effort, the researchers have developed a chemical process that involves doing both at the same time—by converting carbon dioxide into methane, which can be burned to use as a greener energy source.
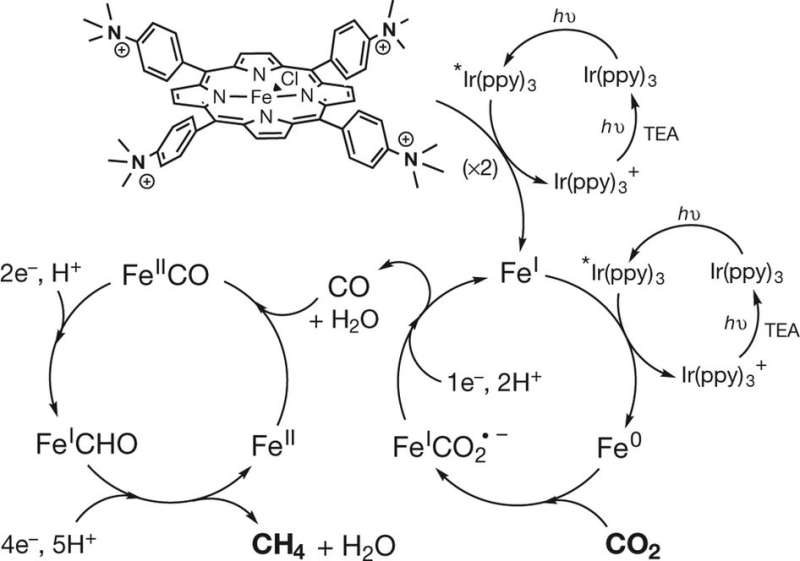
The technique involves irradiating a carbon dioxide solution of acetonitrile, which has a single electron to donate, a photosensitizer and a catalyst that has an iron tetraphenylporphyrin compound that has been functionalized with tetraphenylporphyrin groups. Irradiation by sunlight continues for several hours. The process leads to the creation of methane, carbon monoxide and hydrogen.
The researchers acknowledge that the process is grossly inefficient because the product produced is actually 82 percent carbon monoxide. It is also very slow, producing just 12 grams of methane per hour. But the team believes it can be made much more efficient using a revised two-step procedure. This is because they noticed that what was actually occurring was a conversion of initial ingredients to a mostly carbon monoxide product, some of which was then converted to methane. The researchers note that they also used pure carbon dioxide for their tests, from a canister. An improved process would have to include first pulling the gas from the air, while also keeping out impurities.
The team also has plans to better understand what actually occurs during the reactions—they know the iron binds to the carbon dioxide during the first part of the process, but it is still not clear how hydrogenation of the molecule occurs.
More information: Heng Rao et al. Visible-light-driven methane formation from CO2 with a molecular iron catalyst, Nature (2017). DOI: 10.1038/nature23016
Abstract
Converting CO2 into fuel or chemical feedstock compounds could in principle reduce fossil fuel consumption and climate-changing CO2 emissions. One strategy aims for electrochemical conversions powered by electricity from renewable sources, but photochemical approaches driven by sunlight are also conceivable6. A considerable challenge in both approaches is the development of efficient and selective catalysts, ideally based on cheap and Earth-abundant elements rather than expensive precious metals7. Of the molecular photo- and electrocatalysts reported, only a few catalysts are stable and selective for CO2 reduction; moreover, these catalysts produce primarily CO or HCOOH, and catalysts capable of generating even low to moderate yields of highly reduced hydrocarbons remain rare. Here we show that an iron tetraphenylporphyrin complex functionalized with trimethylammonio groups, which is the most efficient and selective molecular electro- catalyst for converting CO2 to CO known18, 19, 20, can also catalyse the eight-electron reduction of CO2 to methane upon visible light irradiation at ambient temperature and pressure. We find that the catalytic system, operated in an acetonitrile solution containing a photosensitizer and sacrificial electron donor, operates stably over several days. CO is the main product of the direct CO2 photoreduction reaction, but a two-pot procedure that first reduces CO2 and then reduces CO generates methane with a selectivity of up to 82 per cent and a quantum yield (light-to-product efficiency) of 0.18 per cent. However, we anticipate that the operating principles of our system may aid the development of other molecular catalysts for the production of solar fuels from CO2 under mild conditions.
Journal information: Nature
© 2017 Phys.org