This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new method for storing and processing hydrogen chloride
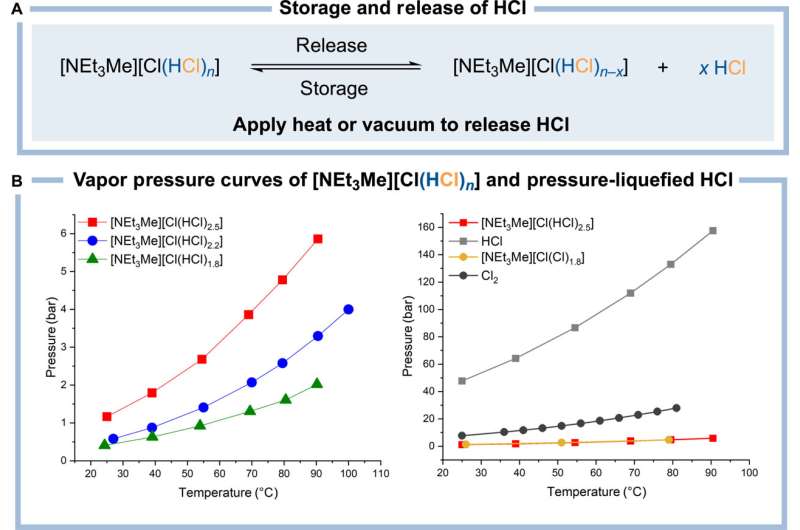
A research team at Freie Universität Berlin has successfully developed a method for storing and electrolyzing gaseous hydrogen chloride in the form of an ionic liquid. The method allows the hydrogen chloride created as a by-product in traditional chlorination processes to be recovered and recycled in a safer manner.
This development also represents an important contribution toward making the chemical industry more sustainable and offering alternative energy solutions. The results of the study were recently published in the journal Science Advances under the title "Bichloride-Based Ionic Liquids for the Merged Storage, Processing, and Electrolysis of Hydrogen Chloride."
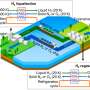
Hydrogen chloride (HCl) is an important by-product of the chemical industry—and can be electrolyzed to generate hydrogen and chlorine. Chlorine is one of the most important base chemicals, required for the production of indispensable polymers such as polyurethanes and polycarbonates, while hydrogen could be the key to the energy of the future.
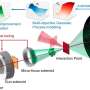
However, for this processing to occur, HCl must first be transported securely from industrial manufacturing plants to special electrolysis facilities powered by green energy. Due to the technically demanding nature of its secure transportation, such applications for HCl have largely been avoided to date. As such, the potential of this valuable resource has only been partially realized thus far.
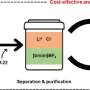
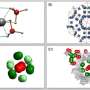
Led by inorganic chemistry professor Sebastian Hasenstab-Riedel and in collaboration with partners from Technische Universität Berlin, a team of researchers at Freie Universität Berlin has developed a secure method of storing HCl. By converting it into an ionic liquid, hydrogen chloride can be handled more safely in its anhydrous (water-free) state.
The researchers discovered that HCl gas can be safely bound to the salt triethylmethylammonium chloride to create an ionic liquid called "bichloride" under ambient conditions. It can then also be safely released from this bichloride following transportation or storage. This approach is not only safer than traditional techniques, its electrolysis also promises to be more energy efficient than traditional systems. Moreover, the bichloride can also be used to synthesize other base chemicals that can then be used to manufacture plastics or silicones.
"Chlorine and hydrogen chloride are gaining in importance when it comes to ushering in a more sustainable future for the energy-intensive chemical industry," says Hasenstab-Riedel. He emphasizes that the technology developed by his team offers ideal conditions for storing and transporting hydrogen chloride in a cost-effective and secure manner.
"This represents an important step toward producing chlorine in a more sustainable way, for example, with electricity produced through renewable energy," says Hasenstab-Riedel. In addition, due to the industrial significance of chlorine, this system could also play an important role in stabilizing the power grid, thus supporting a transition to more sustainable energy practices. The ability to store hydrogen chloride as ionic liquids paves the way for a more sustainable way of performing fundamental chemical processes.
More information: Gesa H. Dreyhsig et al, Bichloride-based ionic liquids for the merged storage, processing, and electrolysis of hydrogen chloride, Science Advances (2024). DOI: 10.1126/sciadv.adn5353
Journal information: Science Advances
Provided by Free University of Berlin