May 18, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Using an organic catalyst to make chlorine more energy efficiently
A team of chemists from Tsinghua University and Guangxi Normal University, both in China, has found an organic catalyst that can be used to make chlorine more energy efficiently. In their paper published in the journal Nature, the group describes how they searched for and found an organocatalyst with an amide functional group that could be used to enable a chlorine evolution reaction.
Thomas Turek, with Clausthal University of Technology, has published a News and Views piece in the same journal issue outlining the work done by the team in China.
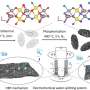
Chlorine (a yellowish-green gas at room temperature) is widely used to disinfect water, such as in swimming pools. But it is also used in a wide variety of other applications such as making plastics and bleaching paper. Unfortunately, the process for producing chlorine uses a lot of energy and also emits a lot of carbon dioxide—it involves electrolysis of sodium chloride solutions that generate both chlorine and sodium hydroxide—both of which are used in manufacturing applications.
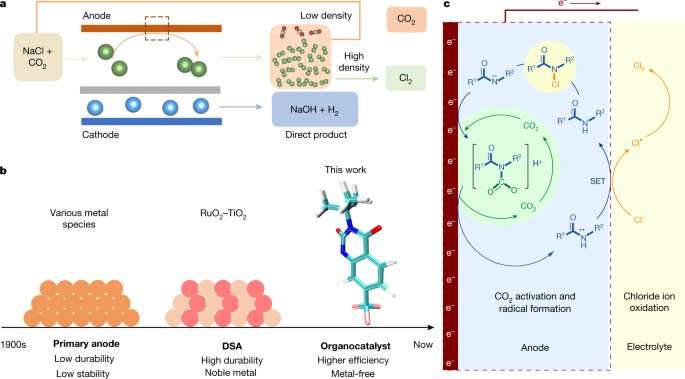
For that reason, scientists have been looking for more efficient and cleaner ways to make the material. One such approach has involved the use of organic (instead of metal) catalysts because they tend to be less expensive, less toxic and they are generally easier to obtain.
After searching for possibilities, the team found an organic molecule in the amide group RCON-H that enabled the creation of chlorine by helping chloride ions in salt water combine in ways that led to the production of chlorine on a positive electrode. They also found that adding CO2 gave rise to the intermediate, carbamic acid, which enhanced efficiency of the catalyst exponentially; this was because it binds to the amide nitrogen and in so doing created a radical species.
They also found that after the reaction, it was relatively easy to separate the CO2 from the chlorine because of the differences in physical properties. Testing showed that the process was able to achieve a density of 10 kA m⁻² and had a selectivity of 99.6% with an overpotential of just 89 mV.
The team concludes by noting that future processes using the new catalyst would use far less energy than the conventional approach and they would be much cleaner—many would not release any harmful by-products at all. They also suggest that their new approach could pave the way in general for a cleaner approach to producing chlorine.
More information: Jiarui Yang et al, CO2-mediated organocatalytic chlorine evolution under industrial conditions, Nature (2023). DOI: 10.1038/s41586-023-05886-z
Thomas Turek, Organic catalyst opens way to energy-efficient chlorine production, Nature (2023). DOI: 10.1038/d41586-023-01583-z
Journal information: Nature
© 2023 Science X Network