A step closer to sustainable energy from seawater
The research group led by Leiden chemist Marc Koper has discovered a catalyst that minimizes the production of chlorine gas during salt water electrolysis. The invention can enable the direct production of hydrogen from seawater. The article has been published in the Journal of the American Chemical Society.
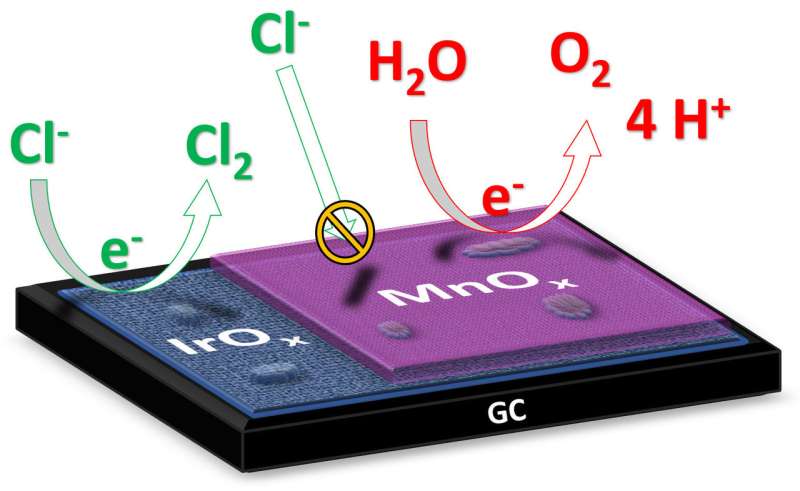
"In the electrolysis of salt water, such as seawater, the ultimate goal is to produce hydrogen at the cathode," explains Ph.D. student Jan Vos from the Leiden Institute of Chemistry. "The product formed at the anode is ideally oxygen, because that is harmless to the environment." However, during salt water electrolysis toxic chlorine gas can also form at the anode. The researchers have now produced a catalyst that minimizes the formation of chlorine gas in favour of oxygen formation. Vos explains: "The catalyst consists of two metal oxides: iridium oxide with a layer of manganese oxide only a dozen nanometers thick. Iridium is a material that exhibits high catalytic activity for the formation of both oxygen gas and chlorine gas; the manganese oxide acts as a kind of membrane that prevents the supply of chloride ions and suppresses the formation of chlorine gas."
The electrolysis of water is an important step for the production and use of hydrogen as an alternative energy carrier. An anode that counteracts the formation of chlorine gas enables water electrolysis where it is not necessary to first rid the water of dissolved salt, the process of which still costs significant amounts of energy and capital. It would allow the direct production of hydrogen from seawater, thereby relieving the rare freshwater reserves on earth.
According to Vos, a useful side effect of salt water electrolysis is the production of very pure fresh water. "If the extracted hydrogen gas is ultimately used as fuel, for example in a fuel cell of a car, the hydrogen reacts back to water with oxygen gas from the atmosphere. That way, the large-scale application of water electrolysis and hydrogen in fuel cells will lead to large quantities of this 'waste product': pure water. In a future where water shortages become an ever more acute problem, this would certainly not be undesirable."
The research sheds new light on a question in chemistry that has been going on for decades. "We originally had no idea why materials based on manganese oxide had such a high selectivity towards oxygen. We assumed that it was purely a catalytic property of the material, but possible effects of diffusion barriers: the selective blocking of the transport of chloride ions... That did not even come to mind! In fact, it is a very basic, effective solution to a very complex problem. That has radically changed our research direction."
The discovery has implications for selectivity in electrolysis. Selectivity is an important criterion for how well a catalytic converter works. In many (electro-)chemical processes, it is possible to form different products during a reaction, but it is hoped for that only the required product is formed. The way to influence selectivity is usually to select the catalyst very precisely and to fine-tune it, but this takes a lot of time and money. Moreover, it is not always possible to combine high selectivity with high activity, another important characteristic.
According to Vos, the research fits nicely into an emerging, alternative trend in electrocatalysis: the use of certain coatings to improve a catalyst. "Such a layer prevents unwanted reactants from reaching the catalyst. This allows an active but non-selective catalytic material to be made selective in an alternative way."
More information: Johannes G. Vos et al. MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution, Journal of the American Chemical Society (2018). DOI: 10.1021/jacs.8b05382
Journal information: Journal of the American Chemical Society
Provided by Leiden University