This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Even far below freezing, ice's surface begins melting as temperatures rise
Physics is filled with mysteries. To find a few worth exploring, look no further than an ice cube. At room temperature, of course, the cube will melt before your eyes. But even far below freezing, ice can shift in barely perceptible ways that scientists are still trying to understand. Using imaging tools at the U.S. Department of Energy's (DOE) Argonne National Laboratory, researchers have detected a phenomenon known as premelting at temperatures far lower than those previously observed.
Their findings are published in the journal Proceedings of the National Academy of Sciences.
Premelting is the reason that a patch of ice can be slippery even on a frigid, clear day. Though the spot is frozen, some part at the surface is wet, an idea first posited by Michael Faraday in the mid-1800s. The idea of a premelted, liquid-like layer on ice opens up other longstanding questions about how water transforms from liquid to solid to vapor—and how, under certain conditions, it can be all three at once.
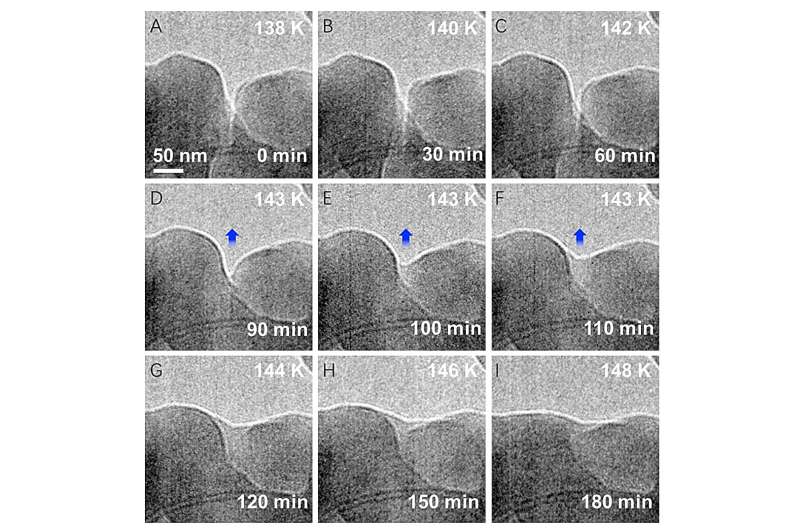
In the recent study, scientists examined ice crystals formed below minus 200 degrees Fahrenheit. The team used Argonne's Center for Nanoscale Materials (CNM), a DOE Office of Science user facility, to grow and observe the ice nanocrystals, which measured only 10 millionths of a meter across.
Besides what the study reveals about the nature of water at subfreezing temperatures, it demonstrates a method for examining sensitive samples in molecular detail: low-dose, high-resolution transmission electron microscopy (TEM). TEM directs a stream of electrons, which are subatomic particles, at an object. A detector creates an image by picking up how the electrons scatter off the object.
"Some materials are beam-sensitive. When you use an electron beam to image them, they can be changed or destroyed," said Jianguo Wen, Argonne materials scientist and a lead author on the paper. One example of an electron beam sensitive material is electrolytes, which exchange charged particles in batteries." Being able to study them in fine detail without disrupting their structure could help in the development of better batteries.
But to start, researchers are experimenting with the low-dose TEM technique on frozen water. After all, water is cheap and abundant. More than that, Wen said, "Ice is very challenging to image, because it is so unstable under the high-energy electron beam. If we successfully demonstrate this technique on ice, imaging other beam-sensitive materials will be a piece of cake."
The low-dose technique combines the CNM's aberration-corrected TEM with a specialized direct electron detection camera. The system is extremely efficient at capturing information from each and every electron that hits a sample, so it is possible to get a high-resolution image using fewer electrons, thus inflicting less damage on the target than a conventional TEM approach.
The low level of electron exposure makes it possible to capture something as delicate as an ice crystal in situ, or in its environment. The research team used liquid nitrogen to grow the ice crystals on carbon nanotubes at 130 degrees Kelvin, or minus 226 degrees Fahrenheit.
Previous studies had observed premelting close to water's triple point. At the triple point, the temperature is just a hair above freezing and the pressure is low enough that ice, liquid and water vapor can exist at once. At temperatures and pressures below triple point, ice sublimates directly into water vapor.
The "rules" of water's behavior are often neatly summed up in a simple phase diagram that maps out water's varying states across different combinations of temperature and pressure.
"But the real world is much more complex than this simple phase diagram," said Tao Zhou, Argonne materials scientist and another corresponding author of the paper. "We showed that premelting can happen far down on the curve, though we cannot explain why."
In a video captured during the experiment, two separate nanocrystals can be seen dissolving into each other as the ice is warmed under constant pressure to 150 degrees Kelvin, or minus 190 degrees Fahrenheit. Though still well below freezing, the ice formed a quasi-liquid-like layer. This ultraviscous water is not accounted for among the simple lines of the phase diagram, where water goes directly from ice to vapor.
The study raises intriguing questions that could be explored in future work. What is the exact nature of the liquid-like layer the researchers saw? What would happen if the pressure is raised, along with the temperature? And does this technique pave the way toward a glimpse of "no-man's land," the state where super-cooled water suddenly crystallizes from liquid into ice? The centuries-long scientific inquiry into water's many states continues.
Co-authors with Wen and Zhou are Lei Yu, Thomas Gage, Suvo Banik, Arnab Neogi, Henry Chan, Xiao-Min Lin, Martin Holt, and Ilke Arslan of Argonne; Yulin Lin and Aiwen Lei of Wuhan University; and Nathan Rosenmann of the University of Illinois at Chicago.
More information: Yulin Lin et al, Surface premelting of ice far below the triple point, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2304148120
Journal information: Proceedings of the National Academy of Sciences
Provided by Argonne National Laboratory