Rapid decompression key to making low-density liquid water
Water makes up more than 70 percent of our planet and up to 60 percent of our bodies.
Water is so common that we take it for granted. Yet water also has very strange properties compared to most other liquids. Its solid form is less dense than its liquid form, which is why ice floats; its peculiar heat capacity profile has a profound impact on ocean currents and climate; and it can remain liquid at extremely cold temperatures.
In addition to ordinary water and water vapor, or steam, there are at least 17 forms of water ice, and two proposed forms of super-cooled liquid water.
New work from Carnegie high-pressure geophysicists Chuanlong Lin, Jesse Smith, Stanislav Sinogeikin, and Guoyin Shen found evidence of the long-theorized, difficult-to-see low-density liquid phase of water. Their work is published by Proceedings of the National Academy of Sciences.
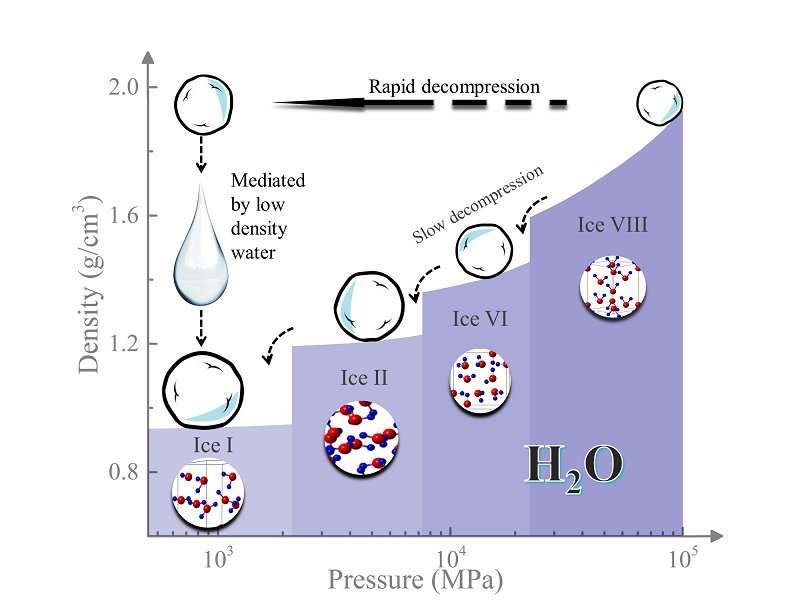
The normal density of water is one gram of water molecules per each cubic centimeter. Studies of anomalies in water's behavior have indicated the existence of liquid water with both lower and higher densities than this standard. But observing these phenomena experimentally has been difficult.
Each molecule has what's called a phase diagram—a sort of chart indicating how its bulk molecular structure changes form under different temperature and pressure conditions. The parts of the phase diagram where low-density water is thought to occur are notoriously difficult to explore, the so-called "water's no-man's land," because they require a path through a series of very specific, very difficult conditions.
But the Carnegie team was able to observe low-density water as an intermediate phase using a newly developed rapid-decompression technique to turn the high-pressure crystalline phase ice-VIII to the diamond-like ice Ic at temperatures between about -207 and -163 degrees Fahrenheit (140 and 165 kelvin).
Sophisticated x-ray analysis confirmed the observation of the low-density liquid water phase, which only lasted for about half a second at -163 degrees Fahrenheit (160 kelvin).
When ice-VIII was decompressed at moderate speeds, it formed other phases of ice, indicating that the speed of decompression is key to observing the low-density liquid water phase.
"Our newly developed, very fast decompression method was the key to this exciting observation of low-density liquid water as an intermediate between two crystalline phases," Shen explained.
More information: Chuanlong Lin et al. Experimental evidence of low-density liquid water upon rapid decompression, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1716310115
Journal information: Proceedings of the National Academy of Sciences
Provided by Carnegie Institution for Science