This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop method to probe supercooled water using electron diffraction
Researchers at EPFL have found a way to study water in "no man's land," a subzero temperature range where water crystallizes rapidly. Historically, the inability to access "no man's land" has prevented scientists from unriddling the anomalous nature of water, but the breakthrough method can now change that.
Water is one of the most essential and widespread compounds on Earth. Covering over 70% of the planet's surface, it has shaped its composition and geology, it regulates its climate and weather patterns, and is at the foundation of all life as we know it.
But water is also weird. It exhibits a number of anomalous properties, of which scientists have identified over seventy—so far. Several theories try to explain these anomalies, but verifying them experimentally is difficult. One of the reasons is that this would require studying water between 160 K and 232 K (-113 °C to -41 °C), a notorious temperature range known as "no man's land" where water crystallizes so fast that it has been impossible for scientists to study its properties.
But why would anyone want to cool water to such low temperatures? Because when water is cooled way below its freezing point it becomes 'supercooled' with unique and fascinating properties; for example, under certain conditions it can remain in liquid form but can freeze instantly when disturbed or exposed to certain substances. Supercooled water is obtained by taking liquid water and cooling it below the freezing point while using tricks to prevent it from crystallizing or at least slowing this process down. However, even with these tricks, crystallization in 'no man's land' is still too fast.
"An experiment to systematically probe the structure of water across so-called 'no man's land' has remained elusive for decades," says Professor Ulrich Lorenz at EPFL's School of Basic Sciences. Now, scientists led by Lorenz have found a way to do just that. The team developed a way to rapidly prepare deeply supercooled water at a well-defined temperature and probe it with electron diffraction before it can crystallize.
"We have still not fully understood why water is an anomalous liquid, despite this topic being hotly debated for over forty years," says Lorenz. "The answer appears to lie in 'no man's land." But because of fast crystallization, any measurement over the full temperature range has not been possible. We do this for the first time. This brings us closer to solving this long-standing mystery."
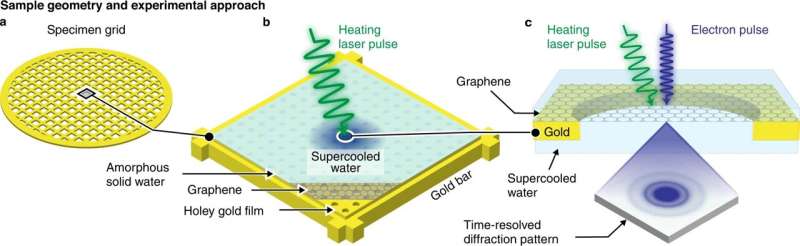
The scientists performed the experiments with a specialized time-resolved electron microscope they custom built in their lab. They prepared the supercooled water at a well-defined temperature and probed it directly before crystallization occurred. To do this, they cooled a layer of graphene to 101 K and deposited a thin film of amorphous ice. They then locally melted the film with a microsecond laser pulse to obtain water in 'no man's land," and captured a diffraction pattern with an intense, high-brightness electron pulse.
The researchers found that as water is cooled from room temperature to cryogenic temperatures, its structure evolves smoothly. At temperatures just below 200 K (about -73 °C), the structure of water begins to look like that of amorphous ice—a form of ice where water molecules are in a disordered state—unlike the tidy crystalline ice we are usually familiar with.
"The fact that the structure evolves smoothly allows us to narrow down the range of possible explanations for the origin of water anomalies," says Lorenz. "Our findings and the method we have developed bring us closer to unriddling the mysteries of water. It is difficult to escape the fascination of this ubiquitous and seemingly simple liquid that still has not given up all of its secrets."
The research is published in the journal Nature Communications.
More information: Constantin R. Krüger et al, Electron diffraction of deeply supercooled water in no man's land, Nature Communications (2023). DOI: 10.1038/s41467-023-38520-7
Journal information: Nature Communications
Provided by Ecole Polytechnique Federale de Lausanne