This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Sodium's high-pressure transformation can tell us about the interiors of stars, planets

Travel deep enough below Earth's surface or inside the center of the sun, and matter changes on an atomic level.
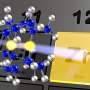
The mounting pressure within stars and planets can cause metals to become nonconducting insulators. Sodium has been shown to transform from a shiny, gray-colored metal into a transparent, glass-like insulator when squeezed hard enough.
Now, a University at Buffalo-led study has revealed the chemical bonding behind this particular high-pressure phenomenon.
While it's been theorized that high pressure essentially squeezes sodium's electrons out into the spaces between atoms, researchers' quantum chemical calculations show that these electrons still very much belong to the surrounding atoms and are chemically bonded to each other.
"We're answering a very simple question of why sodium becomes an insulator, but predicting how other elements and chemical compounds behave at very high pressures will potentially give insight into bigger-picture questions," says Eva Zurek, Ph.D., professor of chemistry in the UB College of Arts and Sciences and co-author of the study, which was published in Angewandte Chemie, a journal of the German Chemical Society. "What's the interior of a star like? How are planets' magnetic fields generated, if indeed any exist? And how do stars and planets evolve? This type of research moves us closer to answering these questions."
The study confirms and builds upon the theoretical predictions of the late renowned physicist Neil Ashcroft, whose memory the study is dedicated to.
It was once thought that materials always become metallic under high pressure—like the metallic hydrogen theorized to make up Jupiter's core—but Ashcroft and Jeffrey Neaton's seminal paper two decades ago found some materials, like sodium, can actually become insulators or semiconductors when squeezed. They theorized that sodium's core electrons, thought to be inert, would interact with each other and the outer valence electrons when under extreme pressure.
"Our work now goes beyond the physics picture painted by Ashcroft and Neaton, connecting it with chemical concepts of bonding," says the UB-led study's lead author, Stefano Racioppi, Ph.D., a postdoctoral researcher in the UB Department of Chemistry.
Pressures found below Earth's crust can be difficult to replicate in a lab, so using supercomputers in UB's Center for Computational Research, the team ran calculations on how electrons behave in sodium atoms when under high pressure.
The electrons become trapped within the interspatial regions between atoms, known as an electride state. This causes sodium's physical transformation from shiny metal to transparent insulator, as free-flowing electrons absorb and retransmit light but trapped electrons simply allow the light to pass through.
However, researchers' calculations showed for the first time that the emergence of the electride state can be explained through chemical bonding.
The high pressure causes electrons to occupy new orbitals within their respective atoms. These orbitals then overlap with each other to form chemical bonds, causing localized charge concentrations in the interstitial regions.
While previous studies offered an intuitive theory that high pressure squeezed electrons out of atoms, the new calculations found that the electrons are still part of surrounding atoms.
"We realized that these are not just isolated electrons that decided to leave the atoms. Instead, the electrons are shared between the atoms in a chemical bond," Racioppi says. "They're quite special."
Other contributors include Malcolm McMahon and Christian Storm from the University of Edinburgh's School of Physics and Astronomy and Center for Science at Extreme Conditions.
The work was supported by the Center for Matter at Atomic Pressure, a National Science Foundation center led by the University of Rochester that studies how pressure inside stars and planets can rearrange materials' atomic structure.
"Obviously it is difficult to conduct experiments that replicate, say, the conditions within the deep atmospheric layers of Jupiter," Zurek says, "but we can use calculations, and in some cases, high-tech lasers, to simulate these kinds of conditions."
More information: Stefano Racioppi et al, On the Electride Nature of Na‐hP4, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202310802
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by University at Buffalo