This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Bringing out the color in zinc to expand its potential properties
Zinc is an important element that is found widely in biological systems, is cheap to manufacture relative to other metals, and has low toxicity. However, unlike other similar metals that exhibit a variety of vibrant colors in metal complexes, seeing different colors for zinc materials was not thought possible.
In a study published in Angewandte Chemie International Edition, researchers from the Institute of Industrial Science, The University of Tokyo, have synthesized a complex with two zinc ions that does exhibit color—greatly expanding the potential properties of zinc complexes.
Dramatic color changes are often used to demonstrate chemical reactions for fun; however, they can also have important uses in indicators, sensing, and smart materials. For certain metal complexes these changes happen because visible light has just the right energy to move electrons between the orbitals—the parts of the atom structure that accommodate the electrons. However, the energy gap between such orbitals of zinc's most stable ion is much larger than the energy of visible light, so the electrons can't be moved between the orbitals—and therefore can't produce color.
Researchers from the Institute of Industrial Science, The University of Tokyo, have now shown that bringing a second zinc atom into play can result in a material that is yellow, both as a solid and when dissolved into solution.
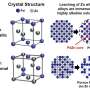
The researchers carefully designed two molecules containing silicon atoms that provided perfect docking stations for the zinc ions to slot into. Both zinc–silyl complexes supported two zinc atoms but at different distances apart.
"We used two systems to show that the zinc atoms work together to create a complex that absorbs light in the visible spectrum," explains lead author of the study Yoshimasa Wada. "In the first system the zinc atoms were relatively far apart—5.71 angstroms—and the material was colorless. While in the second system, they were much closer together—2.93 angstroms—and the zinc material was yellow."
In the system where the zinc atoms were closer together, they were able to combine their orbitals so that the energy needed for their electrons to rearrange was in the visible region. On a large scale this meant that both the solid and solution of the second complex appeared yellow.
"The observed interaction between the zinc centers broadens the potential properties of zinc complexes," says Yusuke Sunada, senior author. "We believe our findings will open up a whole new family of interesting materials."
Zinc can now add visible light interaction to its list of useful properties. Given the prevalence of zinc in biology and its low toxicity, this could open up new uses for zinc in biosensing and biocatalysis.
More information: Yoshimasa Wada et al, Visible Light Responsive Dinuclear Zinc Complex Consisting of Proximally Arranged Two d10‐Zinc Centers, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202310571
Journal information: Angewandte Chemie International Edition
Provided by University of Tokyo