This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New method combines synthetic biology with AI in the cell-free quest for new antibiotics
The rising resistance of bacteria to antibiotics presents an escalating global health risk. Now, researchers at the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany, have combined synthetic biology and artificial intelligence (AI) to develop a more efficient approach to finding and creating new antimicrobial peptides that are effective against a wide range of bacteria. Their paper is published in the journal Nature Communications.
Bioactive peptides play a key role in health and medicine. More than 80 peptide-based drugs are currently in use, all isolated from natural sources. However, antibiotic resistance is estimated to cause more than 1 million deaths worldwide each year. This number is expected to rise to 10 million by 2050, creating an urgent need for novel methods to accelerate the development of new antimicrobials. An untapped potential lies in the non-natural space, where an estimated number of 2,010 to 2,030 different peptides have yet to be explored.
Pipeline for new bioactive peptides
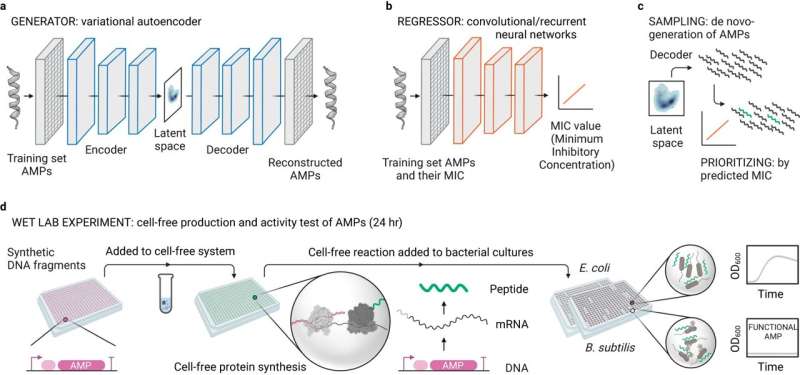
In collaboration with several laboratories at the Max Planck Institute for Terrestrial Microbiology, the University of Marburg, the Max Planck Institute for Biophysics, the Bundeswehr Institute for Microbiology, the iLung Institute and INRAe France, a team of scientists at the Max Planck Institute led by Tobias Erb has established a new pipeline for the development of bioactive peptides.
"In deep learning, a neural network—algorithms inspired by the human brain—learns from large amounts of data. This type of machine learning holds great promise for peptide discovery and de novo design. However, it is usually followed by chemical synthesis of peptides for experimental validation, which is rather difficult and time-consuming and severely limits the number of peptides that can be chemically synthesized," explains Amir Pandi, lead author of the study.
To overcome these limitations, the research team established a cell-free protein synthesis pipeline for the rapid and cost-effective production of antimicrobial peptides directly from DNA templates. The new protocol provides a rapid, low-cost, high-throughput method for antimicrobial peptide screening.
The team first used generative deep learning to design thousands of antimicrobial peptides de novo, and then predictive deep learning to narrow them down to 500 candidates. Among these, screening with the cell-free pipeline identified 30 functional peptides, which the researchers further characterized through molecular dynamics simulations, antimicrobial activity, and toxicity.
Broad-spectrum activity against pathogens
Notably, six of the de novo peptides exhibited broad-spectrum activity against multidrug-resistant pathogens and did not develop bacterial resistance. "We have benefited greatly from this combination of cell-free synthetic biology, artificial intelligence, and a high-throughput approach. By increasing the number of candidates that can be experimentally tested in less than 24 hours, the chance of finding active antimicrobial peptides increased," says Pandi.
"Thus, our cell-free protein synthesis pipeline not only complements recent advances in computational design. It also has the potential to explore the relationship between design and function of bioactive peptides more quickly and cost-effectively."
Tobias Erb adds, "This new method at the interface of synthetic biology and machine learning will be of interest to scientists working in the fields of biomedicine and bioactive peptides."
The next steps include further improving the yield of peptide production as well as employing AI and synthetic biology approaches to design new antimicrobial peptides that are more stable and less toxic, or add a specific mode of action. The researchers also plan to apply augmented deep generative models where the machine learns molecular representations for desired properties, which would improve the success rate of identifying active candidates.
More information: Amir Pandi et al, Cell-free biosynthesis combined with deep learning accelerates de novo-development of antimicrobial peptides, Nature Communications (2023). DOI: 10.1038/s41467-023-42434-9
Journal information: Nature Communications
Provided by Max Planck Society