This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New insights into how cell membranes influence the development of peptides in Alzheimer's disease
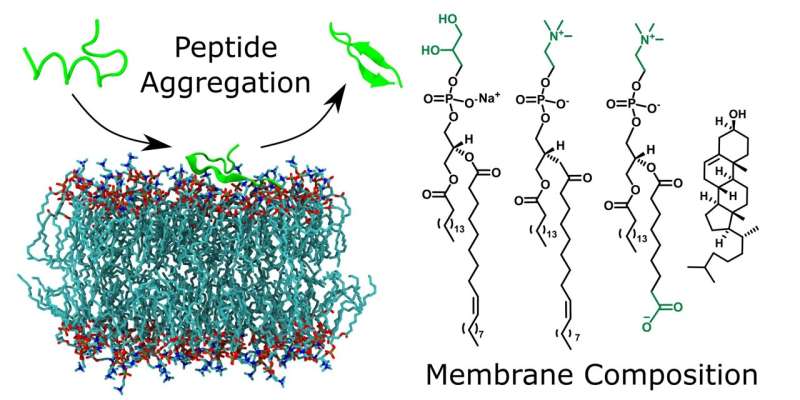
Researchers wanted to better understand how cell membranes in the body influence the structure of peptides—molecules made up of amino acids—and their aggregation. To do this, they used model systems that are easy to control experimentally.
First author Dr. Torsten John, who did his doctorate under Professor Bernd Abel in Leipzig and is now a researcher at the Massachusetts Institute of Technology (MIT) in the US, explains, "One of the effects of stress in the body is that it leads to oxidative processes and thus changes the chemical composition of membranes. In our experiments, we compared the effects of oxidized membranes with those that were not changed."
The scientists combined both biophysical laboratory experiments and computer simulations to better understand peptide aggregation. "Computer simulations, known as molecular dynamics simulations, provide molecular insights into the mechanisms of interactions between membranes and peptides," explains Professor Abel.
It was already known that membrane composition plays an important role in peptide aggregation. However, there has been little research into the role of oxidized membranes. The researchers found that the effects differ between peptides. One of the peptides studied (Aβ40), which is associated with Alzheimer's disease, aggregated faster in the presence of all membranes.
In contrast, the aggregation of another peptide (uperin 3.5) was completely prevented in the presence of the same amount of oxidized membranes. Professor Abel explains, "Depending on the peptide's properties, including its charge, its attraction to the membrane changes, and thus the strength of the influence. If the peptides accumulate on the membrane surface, this accelerates their assembly and aggregation. However, if the attraction is very strong and they change their structure into a helix, then they can no longer aggregate."
The scientists deliberately chose peptides for their study that aggregate similarly but have a different origin. Aβ40 is known to be deposited in the brains of people with Alzheimer's disease, whereas uperin 3.5 is an antimicrobial peptide first discovered in an Australian toad species. Previously, the team of researchers led by Professor Lisandra L. Martin of Monash University in Australia reported on possible links between peptide aggregation in neurodegenerative diseases and the antimicrobial properties of peptides.
The study, published in the journal Chemical Science, further discusses the functional role of amyloid peptides.
More information: Torsten John et al, Lipid oxidation controls peptide self-assembly near membranes through a surface attraction mechanism, Chemical Science (2023). DOI: 10.1039/D3SC00159H
Journal information: Chemical Science
Provided by Leipzig University