This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists design antimicrobial peptides with improved stability
National University of Singapore (NUS) pharmaceutical scientists have successfully applied all-hydrocarbon-stapling modification to improve the enzymatic stability of their previously reported β-hairpin antimicrobial peptides (AMPs) for combating multidrug-resistant bacteria.
There is growing attention on AMPs as an alternative source of antimicrobials to address antibiotic resistance among bacterial infections. Susceptibility to degradation by hydrolytic enzymes in the human body is a major barrier currently limiting their clinical potential. To improve the clinical profile of AMPs, researchers have explored different chemical modification strategies, such as all-hydrocarbon stapling. This strategy has shown promising results on α-helical AMPs, but is relatively underexplored among β-hairpin AMPs.
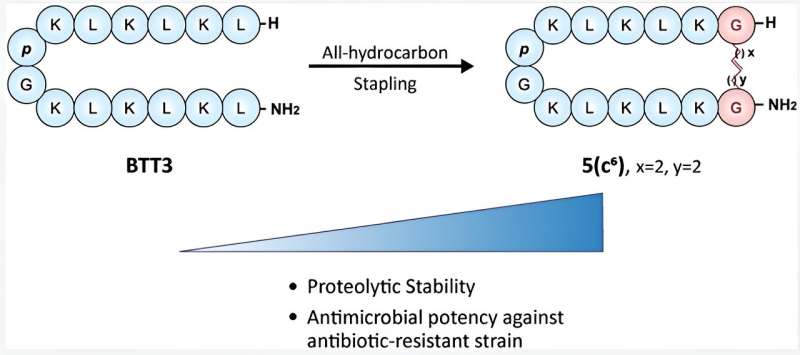
In their previous study, the research team led by Associate Professor Rachel Ee from the Department of Pharmacy, NUS designed a library of synthetic β-hairpin AMPs, from which a particular peptide, BTT3 was identified as a potent candidate.
In this work, now published in the Journal of Medicinal Chemistry, the scientists employed BTT3 as a template sequence to systematically design stapled analogs using all-hydrocarbon stapling by varying the key parameters such as carbon chain length, position of stapling and number of staples. The peptides were functionally and structurally characterized to investigate the impact of the modifications on their clinical profile.
The researchers found that the six-carbon single-stapled peptide 5(c6) displayed the highest proteolytic stability, least toxicity, and had potent antimicrobial activity against antibiotic-resistant clinical strains of Gram-negative pathogens. The stapled peptide 5(c6) was also shown to have a greater bacteria-killing effect than the template peptide BTT3, especially against antibiotic-resistant bacteria.
All-hydrocarbon stapling is a technique to make protein molecules more stable and to stay in a particular shape. In this method, a small, strong "staple" made of hydrocarbon molecules is used to connect two specific points on a molecular chain to hold it together. The stapled peptide can better avoid getting degraded by the enzymes in the human body, hence improving its usefulness as human therapy.
Prof. Ee said, "The findings from this study highlight the potential of the all-hydrocarbon stapling technique for fine-tuning β-hairpin AMPs for clinical applications. By using this technique, medicinal molecules could be made to work better and longer in our bodies. This can be helpful in developing new drugs to effectively combat resistant pathogens to treat various diseases."
More information: Vanitha Selvarajan et al, Stapled β-Hairpin Antimicrobial Peptides with Improved Stability and Activity against Drug-Resistant Gram-Negative Bacteria, Journal of Medicinal Chemistry (2023). DOI: 10.1021/acs.jmedchem.3c00140
Journal information: Journal of Medicinal Chemistry
Provided by National University of Singapore