This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Why you can taste more ethanol in a cold pint of beer or warm glass of baijiu
We all have our own preferred drinking temperatures for different alcoholic beverages, with people commonly enjoying beer or white wine chilled, red wine near room temperature, or baijiu (Chinese whisky) or sake warmed.
In a paper published in the journal Matter on May 1, researchers report that alcoholic beverages may taste more or less "ethanol-like" at different temperatures, and this may be explained by how water and ethanol form either chain-like or pyramid-shaped clusters at the molecular level.
"Two years ago, first author Xiaotao Yang and I were drinking beer together. He had just finished his doctorate degree thesis and asked me, 'what should we do next?'" says lead author and materials scientist Lei Jiang of the Chinese Academy of Sciences.
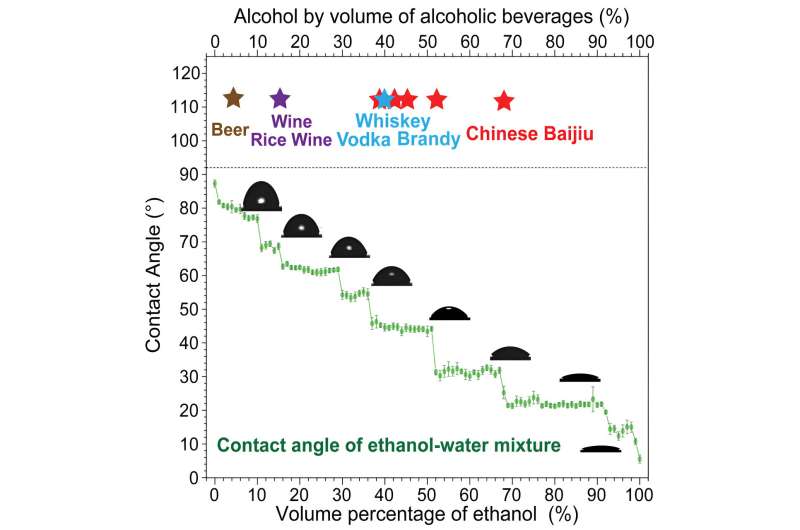
"At the time, I was a scientific committee member of one of the biggest Chinese alcoholic beverage companies, and I had the idea to ask the question 'why does Chinese baijiu have a very particular concentration of alcohol, either 38%–42%, 52%–53%, or 68%–75%?'"
"Then we decided, let's try something, so I put a drop of beer on my hand to see the contact angle," says Jiang.
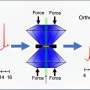
Soon after, Jiang, Yang, and the rest of their team set off to the lab to measure the contact angle of a series of solutions with increasing concentrations of alcohol in water. Contact angle is a common measurement of a liquid's surface tension, and it also tells you how the molecules within the droplet are interacting with each other and with the surface below.
For example, water has a low contact angle on a surface like glass, and so a drop of it will appear "bead-like," where a drop of high alcohol concentration spirits will have a higher contact angle and will instead flatten and spread out.
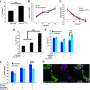
They were surprised to find that the contact angle did not increase linearly with increasing alcohol concentration, but instead the plot showed an irregular series of plateaus as it increased. Additional experiments indicated that this was happening due to the formation of different clusters of ethanol and water in solution.
At low ethanol concentrations, the ethanol forms more pyramid-shaped structures around water molecules; however, when the concentration of ethanol is increased, the ethanol begins to arrange end to end as if in a chain.
Interestingly, the researchers also found that the plateaus that they observed disappeared or appeared when the solutions were cooled or heated, and that some of these trends could explain differences in how alcohol taste is perceived.
For example, 38%–42% and 52%–53% ethanol solutions—like the ethanol concentrations in baijiu—have distinct cluster structures at around room temperature, but this difference disappears at higher temperatures, like 40°C. This could explain why both professional and amateur tasters can distinguish these concentrations of baijiu at room temperature but not at high temperature. At higher temperatures, both concentrations have more chain-like structures and therefore a more "ethanol-like" taste.
"Although there is only 1% difference, the taste of baijiu at 51% and 52% is noticeably different; the taste of baijiu at 51% is similar to that of lower alcohol content, such as 38%–42%. So, in order to achieve the same taste at a lower alcohol content, the distribution of baijiu products ranges most within the 38%–42% and 52%–53% categories," says Jiang.
Similarly, professional testers observe a stronger "ethanol-like" taste in beer after it has been chilled. The results of these experiments show that there is a distinct enhancement in the chain-like structures at 5°C in 5% and 11% ethanol solutions.
"At low temperature, the tetrahedral (pyramid-shaped) clusters become the low concentration amount, and this is why we drink cold beer," says Jiang.
The researchers propose that this information could be leveraged by the alcoholic beverage industry to achieve an "ethanol-like" taste with the lowest ethanol concentration possible.
More information: Ethanol-water cluster determined critical concentration of alcoholic beverages, Matter (2024). DOI: 10.1016/j.matt.2024.03.017. www.cell.com/matter/fulltext/S2590-2385(24)00149-8
Journal information: Matter
Provided by Cell Press