This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New research sheds light on how harmful fungi could become helpful and reduce food waste
Mold and diseases caused by fungi can greatly impact the shelf life of fruit and vegetables. However, some fungi benefit their hosts by aiding plant survival. Colletotrichum tofieldiae (Ct) is a root mold that typically supports continued plant development even when the plant is starved of phosphorus, an important nutrient for photosynthesis and growth. Researchers have studied a unique pathogenic strain of the fungi, called Ct3, which conversely inhibits plant growth. Their research is published in Nature Communications.
By comparing the beneficial and harmful Ct strains, they found that activation of a single fungal secondary metabolism gene cluster determined the negative impact of the fungus on the host plant. When the cluster was disrupted, either genetically or by a change in environment, the fungi's behavior changed from inhibiting growth to promoting it. Understanding mechanisms like this could help us reduce food waste by harnessing the beneficial role fungi can have on food.
When your fresh strawberries go fuzzy with mold, or grapes turn gray and shrivel at the bottom of the fruit bowl, it's always a bit disappointing and unpleasant. The culprit is typically a disease-causing fungus called Botrytis, which devastates food crops globally and is easily spread by wind and soil.
However, there are many fungi that have a less destructive relationship with their host plants, even forming partnerships that can help the plant to thrive. Promoting the beneficial traits of fungi and suppressing undesirable outcomes (like moldy fruit) would greatly aid global food security and help reduce a huge amount of food waste.
"Plant-associated fungi show varied infection lifestyles ranging from mutualistic (beneficial) to pathogenic (harmful) depending on the host environment. However, the mechanisms by which these microbes transit along these different lifestyles remain poorly understood," said Associate Professor Kei Hiruma from the Graduate School of Arts and Sciences at the University of Tokyo.
"We analyzed genetic information from varied strains of a root fungus called Colletotrichum tofieldiae using comparative transcriptomic analysis, which enabled us to study differences in gene expression between each strain. Surprisingly, we found that a single fungal secondary metabolism gene cluster, called ABA-BOT, solely determines whether the fungus exhibits pathogenic or mutualistic traits toward the host plant."
Colletotrichum tofieldae is a fungus that typically benefits plants when they suffer a phosphorus deficiency, helping them thrive despite the lack of this vital nutrient. It has even been shown to increase the growth and yield of economically important crops such as maize and tomatoes. In this study, the multi-institutional team used thale cress as the host plant and sourced six strains of Ct from different geographical locations to infect it.
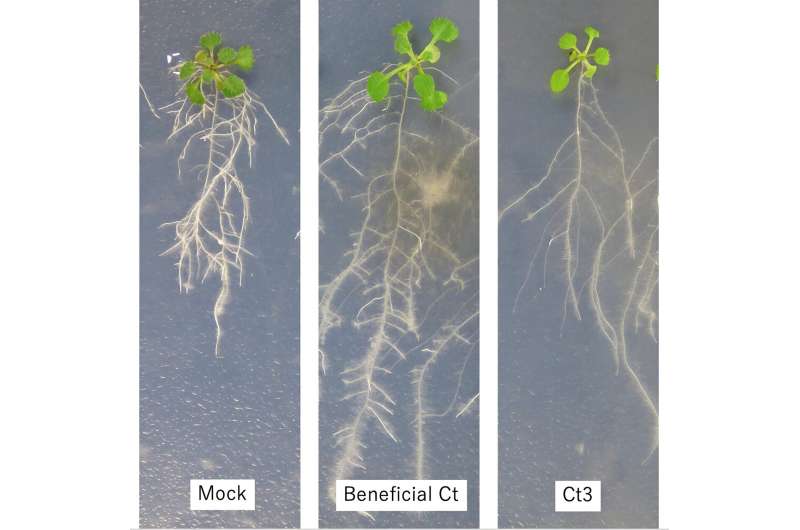
Five strains significantly promoted plant growth, as expected, but a sixth—called Ct3—was found to suppress nutrient uptake, inhibiting plant growth and leading to symptoms of disease. So, what caused this drastic change?
"We identified two key points: First, on the fungal side, that Ct3 activates the ABA-BOT biosynthesis gene cluster; and second, on the plant side, that Ct3 induces the host plant's ABA signaling pathways, through which the fungus inhibits plant growth," explained Hiruma. The researchers found that both pathogenic and mutualistic strains of Colletotrichum tofieldae contain the ABA-BOT gene cluster, but mutualistic strains did not express it, i.e., the genes were not activated.
The discovery came as a surprise, as conventionally pathogens and mutualists were thought to have distinct characteristics, but these findings suggest that they are more intricately related.
When the gene cluster was disrupted, either at a genetic level or by changing the plant's environment, the Ct3 was rendered nonpathogenic and even became beneficial to the host, promoting root growth. Although further study is needed, it appears that the ABA-BOT gene cluster may contribute to pathogenesis in diverse fungi beyond the Ct species.
For example, it may be involved in the pathogenesis of the Botrytis that afflicts our household fruit and vegetables.
"If we gain a comprehensive understanding of the regulatory mechanisms governing the fungal secondary metabolism gene cluster, we can devise a method to selectively suppress potential pathogenesis in otherwise beneficial fungi, optimizing their utilization in agriculture and harnessing the full potential of the microbial diversity naturally present in soil ecosystems," said Hiruma.
"I have come to realize that even pathogens can exhibit nonharmful characteristics during a significant portion of their life cycles. In fact, I am beginning to contemplate the possibility that what we traditionally refer to as pathogens may actually function as beneficial microbes under other conditions."
More information: A fungal sesquiterpene biosynthesis gene cluster critical for mutualist-pathogen transition in Colletotrichum tofieldiae, Nature Communications (2023). DOI: 10.1038/s41467-023-40867-w
Journal information: Nature Communications
Provided by University of Tokyo