This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers decode how bacterium causing 30% of out-of-hospital pneumonia obtains lipids to survive
A research team including the involvement of the UAB has identified the P116 protein, by which the bacterium Mycoplasma pneumoniae obtains cholesterol and other lipids needed to survive. The study, led by the IBMB-CSIC and the Buchmann Institute in Germany, has managed to decipher the structure and functioning of this protein that is so essential to the bacterium. The results have biotechnological implications and will help to develop new tools to fight against Mycoplasma infections.
Mycoplasma pneumoniae is responsible for approximately 30% of community-acquired human pneumonia and other respiratory diseases affecting all ages, but particularly children. This type of bacteria needs to extract lipids, such as cholesterol, from the host environment for survival.
Now, new research has identified the protein with which the M. pneumoniae bacteria extracts cholesterol and other lipids needed to maintain the integrity of its membrane and ensure its survival.
The study, which included the involvement of Marina Marcos and Jaume Piñol, researchers from the UAB Department of Biochemistry and Molecular Biology, was led by researchers Ignacio Fita and Achilleas Frangakis, the former from the Institute of Molecular Biology of Barcelona of the Spanish National Research Council (IBMB-CSIC) and the latter from the Buchmann Institute in Germany.
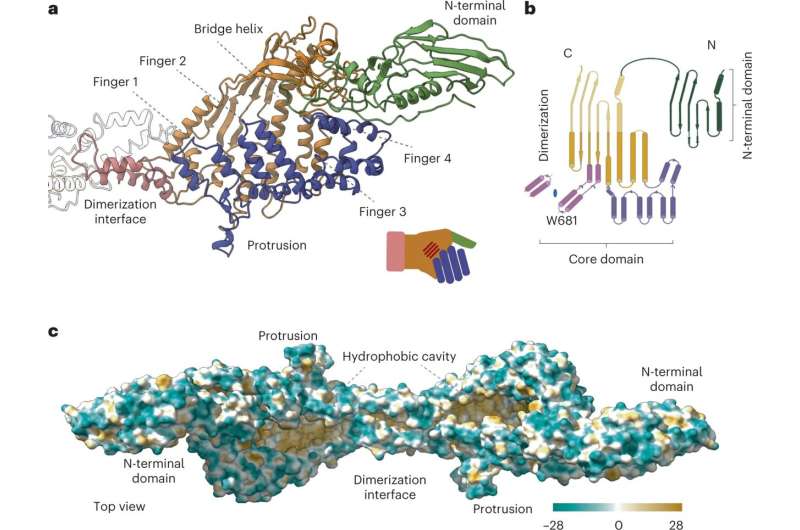
The study, which was published in the journal Nature Structural & Molecular Biology, describes the structure and functioning of the P116 protein by using advanced cryo-electron microscopy technology, which revealed a new structure of this protein that, as Ignacio Fita details, "contains a huge hydrophobic cavity capable of storing different lipid compounds."
The technology of mass spectrometry and the use of radioisotopes have allowed researchers to analyze with great precision the different essential lipids that can be stored in the P116 protein's cavity. The analyses conducted by the research team show that the P116 can extract lipids such as phosphatidylcholine, sphingomyelin and cholesterol, and that it can even obtain cholesterol from the lipoproteins circulating in the host's blood.
"These results can open new possible therapeutic ways to block the mechanism of the P116 protein and, therefore, the infectiousness of Mycoplasma pneumoniae. It also points to several applications in biotechnology," says David Vizarraga, researcher at the IBMB-CSIC, and one of the first authors of the study alongside Lasse Sprankel from the Buchmann Institute in Germany.
More information: Lasse Sprankel et al, Essential protein P116 extracts cholesterol and other indispensable lipids for Mycoplasmas, Nature Structural & Molecular Biology (2023). DOI: 10.1038/s41594-023-00922-y
Journal information: Nature Structural & Molecular Biology
Provided by University of Barcelona