This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Differential dynamics and direct interaction of bound ligands with lipids in multidrug transporter ABCG2
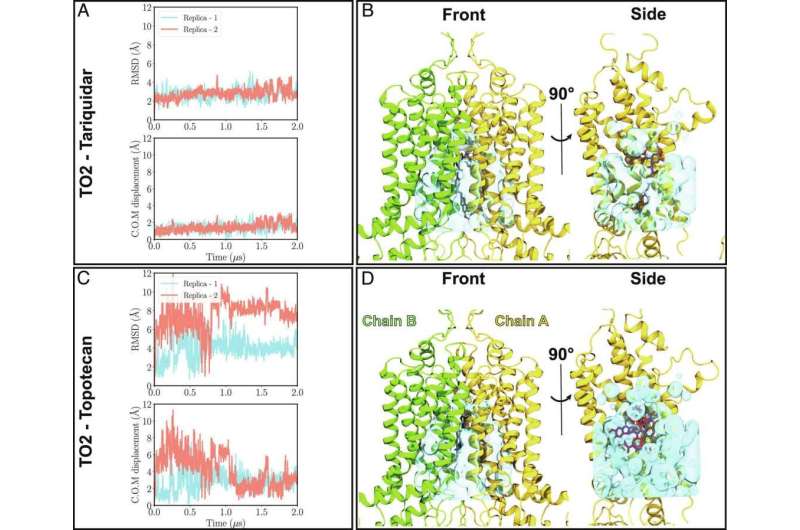
ABC transporters constitute a major class of multidrug resistance proteins, e.g., in cancer cells. Membrane lipids have been long known to influence the structure and functional dynamics of these proteins. However, previous structural and biochemical studies on ABCG2 are limited either by their static description or low spatial resolution to provide a microscopic view of the dynamics of the drug binding and lipid interactions.
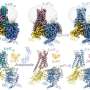
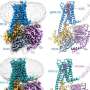
Here, using cryo-EM studies of ABCG2, a prominent member of the ABC family, in its ligand-bound form and in its native turnover state, combined with extended molecular simulations, researchers at University of Illinois and ETHZ show that lipids as well as the ligand size can impact the drug-binding mode of ABC transporters.
They demonstrate that the size of the substrate as a major determinant of its binding stability in ABCG2 drug binding pocket and phospholipids from the cellular membrane can penetrate into the protein and directly interact with bound drugs. They also provide evidence for differential behavior of cholesterol which is not transported by ABCG2 but ironically needed for its proper transport function. These molecular findings have direct functional ramifications on ABCG2's function as a transporter.
The work is published in the journal Proceedings of the National Academy of Sciences.
More information: Ali Rasouli et al, Differential dynamics and direct interaction of bound ligands with lipids in multidrug transporter ABCG2, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2213437120
Journal information: Proceedings of the National Academy of Sciences
Provided by ETH Zurich