This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists map 3D structure and electronic properties of important biological catalyst
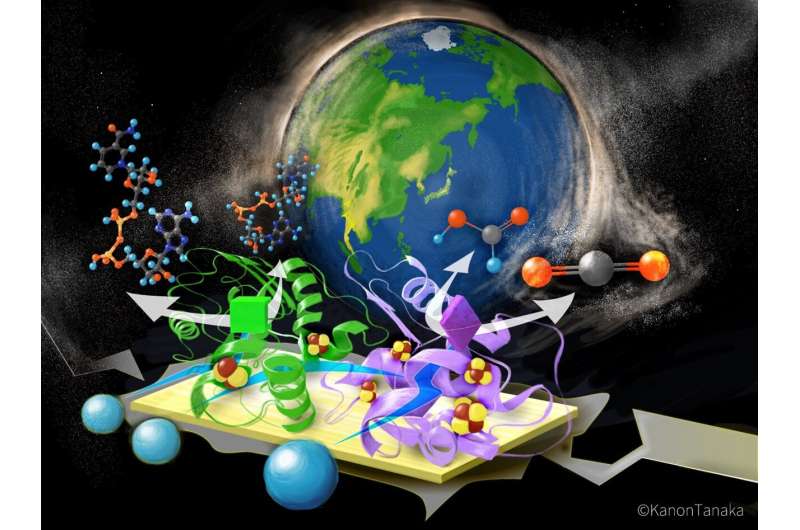
Making atoms and electrons behave according to researchers' intentions is no small task, but scientists often get a little help from nature.
Enzymes from living organisms are well-known for effortlessly directing the buildup and breakdown of molecules in ways that would be difficult or even impossible by conventional chemistry. Putting these biological catalysts to work in industry and health care settings saves time, costs, and even lives.
One such enzyme—FoDH1—is showing great potential for unlocking future carbon capture technologies. Derived from the bacteria Methylorubrum extroquens, this enzyme has the unusual ability to do chemistry with one-carbon molecules, like carbon dioxide. However, its unique electronic properties have left scientists puzzling over its mechanism.
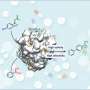
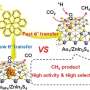
Now a team of Japanese scientists led by Kyoto University has mapped the three-dimensional structure and electronic properties of FoDH1 in unprecedented detail. For the first time, they reveal a unique structure with two sites available for electrons to transfer through metals in the enzyme.
"What's perhaps even more interesting is that FoDH1 can accept and donate electrons directly, without any other mediating chemicals. This could make it a potential bridge between biological systems and electronic devices," says corresponding author Keisei Sowa.
In previous work, Sowa's team hooked the enzyme up to an electrode and demonstrated its unique ability to handle electrons. To further understand how and where electrons were interacting with the enzyme, they examined a single particle of FoDH1 at ultra-low temperatures, revealing the locations of key components, including active clusters of iron and sulfur distributed throughout the enzyme's structure.
After FoDH1 was probed from different sides with specialized electrodes tipped with gold nanoparticles, signals from two separate sites were unexpectedly detected. Analytical calculations pointed to specific clusters containing different numbers of iron and sulfur atoms that make up the electrode-active sites.
"Our results may be the first clear evidence of two electrode-active sites operating in any enzyme, prompting potential uses of FoDH1 in a range of electrocatalytic processes, including CO2 capture," reflects Sowa.
"For example, we may be able to see how changing the structure of a mutated enzyme might allow us even greater control of FoDH1's features."
The paper, "Multiple electron transfer pathways of tungsten-containing formate dehydrogenase in direct electron transfer-type bioelectrocatalysis," was published on April 29, 2022 in the journal Chemical Communications.
More information: Tatsushi Yoshikawa et al, Multiple electron transfer pathways of tungsten-containing formate dehydrogenase in direct electron transfer-type bioelectrocatalysis, Chemical Communications (2022). DOI: 10.1039/D2CC01541B
Journal information: Chemical Communications
Provided by Kyoto University