Researchers explore enzymes that use a cation, not oxygen-addition, to drive reactions
Researchers from North Carolina State University and the University of Texas at Austin have defined the structure of a substrate-bound iron 2-oxoglutarate (Fe/2OG) enzyme to explore whether these enzymes could be used to create a wide array of molecules. They probed the enzyme's active site to determine its ability to bind with different substrates. Additionally, rather than oxygen-addition, they saw that Fe/2OG enzymes likely utilize cations—highly reactive species—to drive desaturation during catalysis. The work, published in Nature Communications, could lead to the use of Fe/2OG enzymes in making a wide array of valuable molecules.
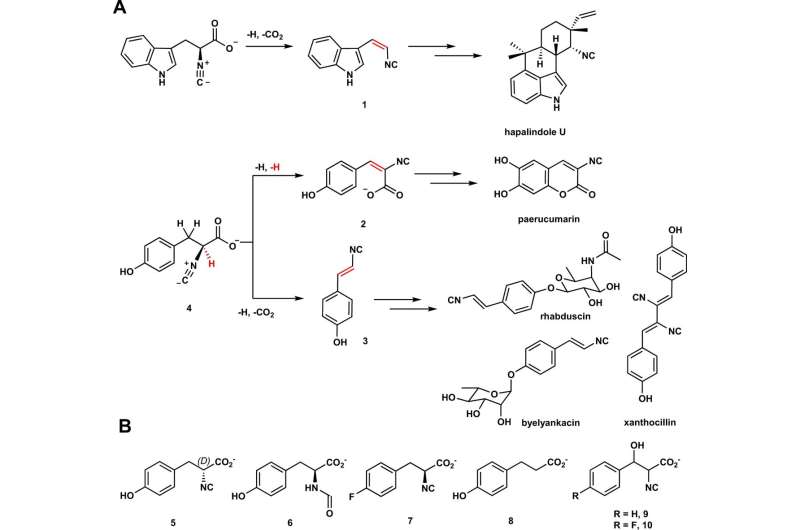
Enzymes in the Fe/2OG family are naturally occurring—they are found in everything from bacteria to plants and animals. As such, these enzymes have the potential to be used as a greener, more efficient platform for creating molecules such as vinyl isonitriles, which have antibiotic properties. However, the pathways by which Fe/2OG enzymes create these molecules are poorly understood.
"The endgame is to understand how the enzymes in this family create particular molecules, so that we can potentially piggyback on a natural process that current chemistry cannot replicate," says Wei-chen Chang, associate professor of chemistry at North Carolina State University and co-corresponding author of a paper describing the work. "So we looked at a couple of different enzymes within the Fe/2OG family to see how they performed different transformations using the same substrate, or molecule they bind to."
By focusing on how the enzyme binds to a particular substrate, the researchers can determine which other substrates could be used by the enzyme, a more efficient way to determine potential reactions and products than experimentation.
The research team focused on two Fe/2OG enzymes—PvcB and PlsnB—and compared their structures and the products they made. They identified binding sites on both enzymes, but when it came to exploring how the enzyme performed its transformations, they made a surprising find.
"Normally, the way Fe/2OG enzymes catalyze or create a new product happens like this: the enzyme binds to the substrate, a single oxygen atom from molecular oxygen (O2) is introduced into the substrate, and oxygen-addition drives the reaction," Chang says. "That process is called hydroxylation.
"But for these enzymes, the transformation or reaction isn't driven by hydroxylation, but by a reactive cation that triggers the subsequent desaturations, where new bonds are introduced."
The two Fe/2OG enzymes studied (PIsnB and PvcB) utilized fundamentally distinct desaturation to create different products from the same substrate.
"Now we know how these enzymes catalyze transformations and have found the binding sites, we have a foundation for determining what they can do in terms of reactions," Chang says. "We can also recommend and predict the best substrates to use to get targeted products."
More information: Wantae Kim et al, Elucidation of divergent desaturation pathways in the formation of vinyl isonitrile and isocyanoacrylate, Nature Communications (2022). DOI: 10.1038/s41467-022-32870-4
Journal information: Nature Communications
Provided by North Carolina State University