Study shows new way for simple production of vicinal diamines

Among the most common structures relevant to the function of biologically active molecules, natural products and drugs are so-called vicinal diamines—in particular, unsymmetrically constructed diamines. Vicinal diamines contain two functional atomic groups responsible for the substance properties, each with a nitrogen atom bonded to two neighboring carbon atoms.
A team led by Prof. Frank Glorius of the Institute of Organic Chemistry at the University of Münster has now presented a new, direct way to produce vicinal diamines. The research is published in the journal Nature Catalysis.
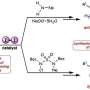
In contrast to other, less suitable methods, the process does not require the use of transition metals and iodine reagents as catalysts. Instead, the researchers use light energy to produce the desired diamines from various electron-rich aromatic hydrocarbons (arenes and heteroarenes).
"In this way, we obtain a series of vicinal diamines that were previously difficult to produce. In doing so, we can precisely control the sites where the functional groups are located," explains first author Dr. Guangying Tan.
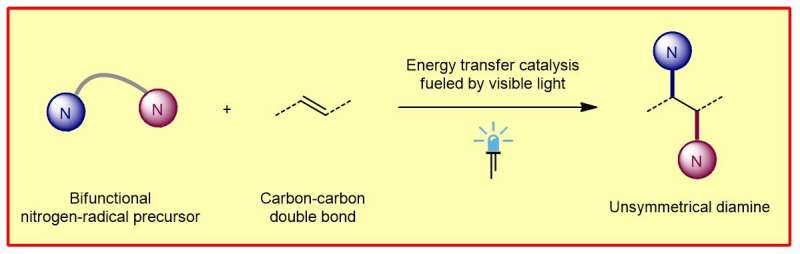
To this end, the chemists developed a class of special nitrogen radical precursors that simultaneously generate two nitrogen-centered radicals with different reactivities via an energy transfer process. By "regioselectively" adding two of these radicals stepwise via carbon-carbon double bonds, the scientists produce the unsymmetrically constructed vicinal diamines.
"Regioselective" means that the reaction occurs at defined sites on the molecules. The functional groups (amino groups) can then be further modified. The fact that the diamines synthesized in this way are not symmetrical, in contrast to a symmetrical structure, opens up a much greater variety of functional groups to be considered.
"The molecules of life consist largely of carbon chains and rings of varying size and complexity. The decoration of these 'plain' chains with other elements is crucial for the resulting properties of these compounds," Frank Glorius explains. The elements oxygen and nitrogen play a key role. Chemists refer to these non-carbon elements as heteroatoms.
"Methods for the efficient and controlled introduction of these heteroatoms into artificially produced, biologically active structures are therefore of great importance," Glorius says. "This also applies to the vicinal diamines we are focusing on."
The chemists perform the diamination reaction under irradiation with blue light-emitting diodes (LEDs) and using an inexpensive and commercially available thioxanthone as an organic photosensitizer.
More information: Guangying Tan et al, Energy transfer-enabled unsymmetrical diamination using bifunctional nitrogen-radical precursors, Nature Catalysis (2022). DOI: 10.1038/s41929-022-00883-3
Journal information: Nature Catalysis
Provided by University of Münster