Chemists discover new reactivity of strained molecules

In synthetic organic chemistry, so-called cycloadditions are a particularly important class of reactions. With this type of reaction, ring-shaped molecules can be constructed simply and efficiently by joining ("adding") two compounds that each contain double bonds. A team led by Prof. Dr. Frank Glorius from the University of Münster has now succeeded in performing an unconventional cycloaddition in which a carbon-carbon double bond reacts with a carbon-carbon single bond. In double bonds, atoms are connected by two pairs of electrons; in single bonds, only one pair of electrons is involved. The key to success was the use of particularly "strained" single bonds. To enable mild reaction conditions, the chemists used a photosensitizer, a catalyst that drives the reaction using light energy. The study has now been published in the journal Nature.
"In addition to its conceptual and mechanistic importance, this method also has a synthetic benefit," explains lead author Roman Kleinmans. "This is because we can use it to build polycyclic, three-dimensional carbon scaffolds that have been difficult or impossible to access. Such three-dimensional architectures are fascinating and play an increasingly important role in medicinal chemistry."
The chemistry in detail
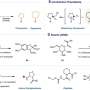
For more than a century, so-called [2+2] photocycloadditions have been studied and developed. Research has focused specifically on [2π+2π]-systems in which, for example, two double bonds react to form two new single bonds giving a four-membered ring product. The team from Münster has now achieved a breakthrough by realizing this type of reaction using a single bond ("2π+2σ").
The team used a class of compounds with "strained" single bonds: so-called bicyclo[1.1.0]butanes (BCBs). These carbon compounds have a butterfly-like shape, with two linked triangles on the single bond that look like wings. The internal wing angles (60 degrees each) deviate greatly from the ideal unstrained angles (109 degrees each). The opening of the central single bond releases strain energy—thermodynamically favoring the reaction with the carbon-carbon double bond. Using this strategy, the researchers also succeeded in incorporating a nitrogen atom into the carbon skeleton of the product.
The visible light driven "triplet-triplet energy transfer catalysis" allows the reaction to be carried out as mildly as possible, so that irradiation of the reaction with harsh UV light, which is commonly used in chemistry of this type, was not needed. In this mechanism, the catalyst absorbs energy from the irradiated light and transfers it to a suitable substrate. The catalyst returns to the ground state (it is regenerated), and the corresponding molecule is left in an energetically excited state (triplet state). The excited molecule is then able to react with the single bond. "We used a simple organic photosensitizer for this, namely thioxanthone, dispensing with rare and expensive transition metal-based catalysts," emphasizes Frank Glorius. To further understand the molecular mechanism of the reaction in more detail, the chemists carried out computational calculations using "density functional theory."
More information: Roman Kleinmans et al, Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer, Nature (2022). DOI: 10.1038/s41586-022-04636-x
Journal information: Nature
Provided by University of Münster