Turning to the laws of physics to study how cells move

Scientists have long been concerned with trying to understand how cells move, for example in pursuit of new ways to control the spread of cancer. The field of biology continues to illuminate the infinitely complex processes by which collections of cells communicate, adapt, and organize along biochemical pathways.
Turning to the laws of physics, researchers at the Yale Systems Biology Institute have taken a fresh look at how cells move, revealing similarities between the behavior of cell tissue and the simplest water droplets.
"We take a different perspective on how cell motion is determined by the properties of the tissues they're in rather than how they act individually," said Michael Murrell, associate professor of Biomedical Engineering and Physics and senior author of a series of papers describing the work.
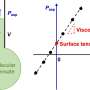
Published in Physical Review Letters, the group's initial experiments used mechanical techniques to measure the surface tension of a simple "ball" of cell tissue to reveal similarities with the thermo-dynamic properties of water droplets, but with noticeable differences.
"With a water droplet the surface tension is constant and doesn't change with droplet size," said Murrell. However, the scientists found that in the case of a "droplet" of cancer cells surface tension was size dependent—the smaller the tissue the higher the surface tension, and the higher the pressure within the tissue.
Next, the team applied a surface tension gradient to show that cells within the tissue moved rapidly and collectively, much like the way the surface of water moves when detergent is added. Their findings were published in Physical Review Fluids.
This so called "Marangoni" effect occurs when the forces at the surface of a tissue drive the motion of cells inside.
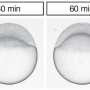
To complete the puzzle, the scientists allowed the tissue to adhere to a surface, mimicking the way a tumor grows and spreads. Cells emerged from the ball of tissue like water droplets "wetting" a receptive—or hydrophilic—surface. In some conditions, the wetting increased the internal pressure of the tissue, helping to push cells out.
Published today in Physical Review X, these findings cast new light on the degree to which cells "migrate" or whether pressure from surface tension promotes cell movement.
"When you think of anything that flows, we usually think about a pressure gradient," said Vikrant Yadav, a research scientist in the Murrell Lab and co-first author of all three studies. "What we show here is that the bulk properties of tissue, including the surface tension and pressure, matter when it comes to the ability of cells to migrate out of a model tumor."
More information: M. S. Yousafzai et al, Active Regulation of Pressure and Volume Defines an Energetic Constraint on the Size of Cell Aggregates, Physical Review Letters (2022). DOI: 10.1103/PhysRevLett.128.048103
Vikrant Yadav et al, Gradients in solid surface tension drive Marangoni-like motions in cell aggregates, Physical Review Fluids (2022). DOI: 10.1103/PhysRevFluids.7.L031101
Muhammad Sulaiman Yousafzai et al, Cell-Matrix Elastocapillary Interactions Drive Pressure-Based Wetting of Cell Aggregates, Physical Review X (2022). DOI: 10.1103/PhysRevX.12.031027
Journal information: Physical Review Letters , Physical Review X
Provided by Yale University