For sustainable sulfur-tolerant catalysts, alloy precious metals with phosphorus
Catalysts play crucial roles in chemical processes. However, many conventional catalysts have suffered from deactivation caused by sulfur-containing molecules which are strongly absorbed onto catalyst surfaces and suppress catalytic reactions. Osaka University researchers have developed a highly active and durable metal-phosphide catalyst for the deoxygenation of sulfoxides. The developed catalyst has a high durability against sulfur-poisoning in contrast with the conventional metal catalysts. Their findings are published in JACS Au.
Transformation of sulfur-containing molecules is a fundamental and significant reaction in organic and pharmaceutical chemistry. However, the sulfur atom strongly coordinates with the active sites of metal catalysts, significantly decreasing the catalytic performance. Sulfur impurities contained in chemical feedstocks also cause catalyst deactivation. Therefore, the development of a new sulfur-tolerant and highly active catalyst is desired.
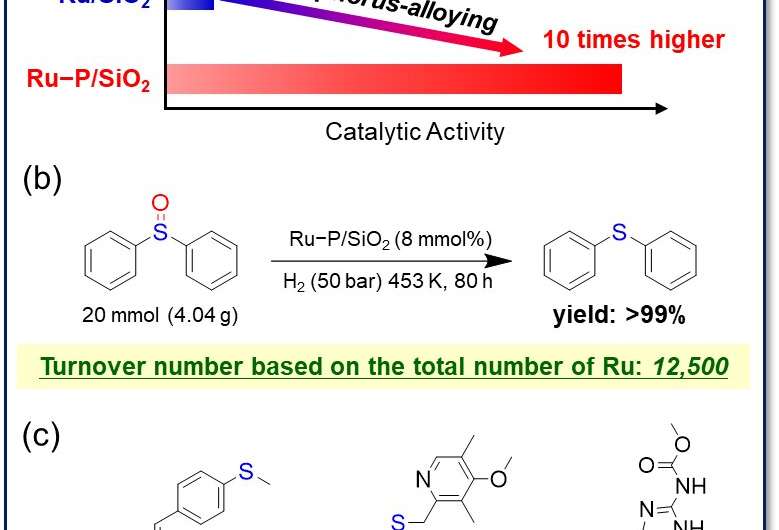
The researchers prepared precious metal phosphide nanoparticle catalysts for the deoxygenation of sulfoxides into sulfides. The integration of phosphorus into the metal framework—a method called "phosphorus-alloying"—greatly improved the catalytic performance of precious metal nanoparticles. In particular, the ruthenium phosphide nanoparticles (Ru−P/SiO2) exhibited excellent catalytic activity and durability against sulfur-poisoning.
"Integration of phosphorus into ruthenium nanoparticles drastically enhanced catalytic activity and durability (Fig. 1a)," study first author Hiroya Ishikawa explains.
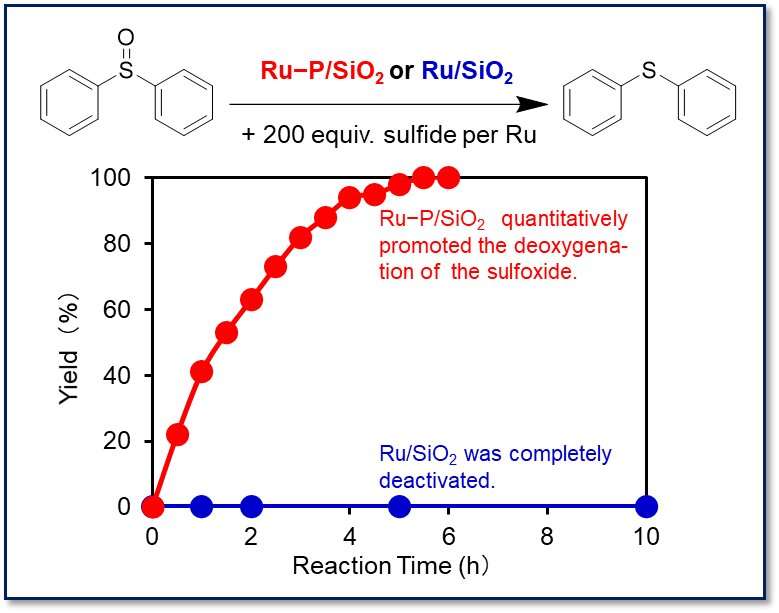
Ru−P/SiO2 achieved the highest turnover number (12,500) reported to date (Fig. 1b). This catalyst shows wide substrate applicability and can deoxygenate structurally complex drug intermediates to produce bioactive sulfides such as sulindac sulfide (anti-inflammatory drug), ufiprazole (anti-ulcer drug), and fenbendazole (anthelmintic) in high yields (Fig. 1c). Moreover, Ru−P/SiO2 can promote sulfoxide deoxygenation even in the presence of a lot of sulfur-containing molecules; even in the presence of 200 equiv. of sulfide per ruthenium, Ru−P/SiO2 quantitatively promoted the deoxygenation of sulfoxide, while the conventional ruthenium nanoparticle catalyst (Ru/SiO2) was completely deactivated (Fig. 2).
"We expect that our metal phosphide catalyst will make a significant contribution to a lot of chemical processes, which suffered from catalyst deactivation caused by sulfur," says study corresponding author Takato Mitsudome. "But beyond this, we believe phosphorus-alloying can be a powerful method for designing highly active and durable metal nanoparticle catalysts for a variety of organic syntheses."
More information: Hiroya Ishikawa et al, Phosphorus-Alloying as a Powerful Method for Designing Highly Active and Durable Metal Nanoparticle Catalysts for the Deoxygenation of Sulfoxides: Ligand and Ensemble Effects of Phosphorus, JACS Au (2022). DOI: 10.1021/jacsau.1c00461
Provided by Osaka University