Nickel phosphide nanoparticle catalyst is the full package
Most catalysts that promote the conversion of glucose to sorbitol offer certain properties while requiring compromises on others. Now, researchers from Osaka University have reported a hydrotalcite-supported nickel phosphide nanoparticle catalyst (nano-Ni2P/HT) that ticks all the boxes. Their findings are published in Green Chemistry.
Sorbitol is a versatile molecule that is widely used in the food, cosmetics, and pharmaceuticals industries. There is therefore a pressing need to produce sorbitol in a sustainable, low-cost, and green manner.
The nickel catalysts that are commonly used in the industrial hydrogenation of glucose to sorbitol are unstable in air and require hash reaction conditions. Rare metal alternatives—despite being more efficient—can be expensive and are susceptible to poisoning.
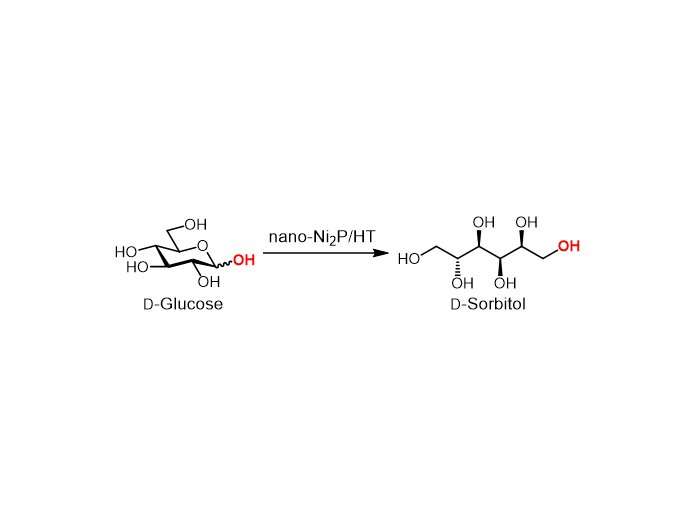
Nano-Ni2P/HT is stable in air and has a high activity for the hydrogenation of glucose to sorbitol. In addition, nano-Ni2P/HT produces a particular sorbitol structure, known as D-sorbitol, at more than 99% yield. This high selectivity means that a high-purity product can be obtained.
The nano-Ni2P/HT-catalyzed hydrogenation can be carried out in water. Moreover, the catalyst shows good conversion and selectivity when the temperature is just 25°C—compared with 100–180°C for conventional processes—or when the hydrogen gas pressure is only 1 bar—compared with 50–150 bar. The energy saved by using these mild conditions would lead to greener and more sustainable procedures, as well as reduce operating costs.
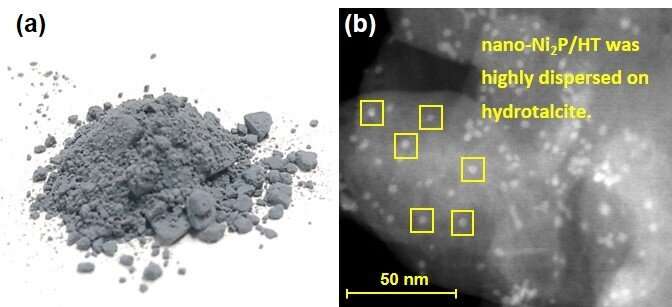
"Our nano-Ni2P/HT catalyst outperformed conventional nickel alternatives in terms of both the catalytic activity and the amount of D-sorbitol that was produced, which is very encouraging," study first author Sho Yamaguchi explains. "nano-Ni2P/HT also gave a better yield of D-sorbitol than a commercially available noble metal catalyst."
Repeated use of the catalyst showed that nano-Ni2P/HT could be recycled with no significant loss of performance. The reaction could also be carried out at high glucose concentration (50 wt%), which demonstrates the viability of the catalyst for large scale use.
"The continual improvement of industrial catalyst is necessary to achieve sustainable, low-cost production with an environmental conscience," says study corresponding author Takato Mitsudome. "We believe our catalyst will make an important contribution, not only to D-sorbitol production, but to the development of other processes that support the pharmaceutical, food, and cosmetics industries."
More information: Sho Yamaguchi et al. Air-stable and reusable nickel phosphide nanoparticle catalyst for the highly selective hydrogenation of d-glucose to d-sorbitol, Green Chemistry (2021). DOI: 10.1039/D0GC03301D
Journal information: Green Chemistry
Provided by Osaka University