All of the performance, none of the fuss: nitrile hydrogenation done right
The need to be mindful consumers is becoming a priority for an ever-growing portion of society. This means that achieving efficient and environmentally sustainable chemical processes is more important than ever before. One way of influencing reaction efficiency is catalysis. However, when choosing a catalyst there is often a need to trade-off different factors including performance and cost. Osaka University researchers have reported a nano-cobalt phosphide catalyst for the hydrogenation of nitriles that combines efficiency, cost effectiveness, ease of handling, and reusability. Their findings were published in Chemical Science.
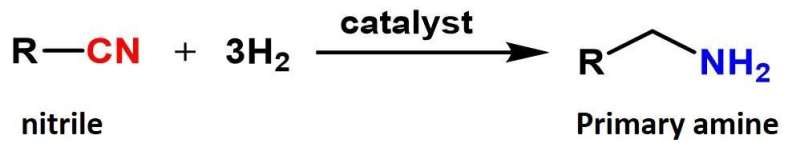
The hydrogenation of nitriles to primary amines is an important process that provides the building blocks for many everyday products and fuels. Primary amines are used as solvents and surfactants as well as in procedures for making dyes, pharmaceuticals, and plastics.
Optimizing nitrile hydrogenation in the interest of cost and environmental sustainability has led to numerous different types of catalyst being reported. Earth-abundant metal catalysts are cost effective—owing to the wide availability their name suggests—but lack air stability, making them difficult to handle. In contrast, precious metal catalysts can be used under mild conditions, but are prohibitively expensive for large scale processes.
The researchers have therefore developed a non-metal alloy heterogeneous cobalt phosphide catalyst that forms nanoparticles (nano-Co2P) that are stable in air and achieve efficient hydrogenation under mild conditions. Crucially, nano-Co2P can also be separated and reused for subsequent reactions.
"Despite being stable in air, our nano-cobalt phosphide catalyst has a very high activity," study author Min Sheng explains. "Its turnover number—which provides a measure of how productive a catalyst is—is 58,000. To put this in context, this is an improvement of up to 500-fold on previously reported catalysts for this type of reaction."
Using the nano-Co2P catalyst, hydrogenation reactions could be carried out using hydrogen gas at ambient pressure, thus making nano-Co2P the first earth metal catalyst to be successfully used under mild conditions. This offers numerous advantages in terms of cost and safety. In addition, the catalyst was found to be effective for hydrogenating nitriles in a wide range of different organic molecules.
"Our study is the first example of a metal phosphide air-stable heterogeneous catalyst being used for this kind of reaction," study lead author Takato Mitsudome explains. "We believe that our findings will inspire a new direction in the catalysis of synthetic processes, supporting sustainable practices that protect the environment."
More information: Takato Mitsudome et al. A cobalt phosphide catalyst for the hydrogenation of nitriles, Chemical Science (2020). DOI: 10.1039/D0SC00247J
Journal information: Chemical Science
Provided by Osaka University