A ruthenium-based catalyst with highly active, flat surfaces outperforms metal-based competitors
Scientists in Japan and India have developed a reusable, high-performance catalyst based on flat-shaped ruthenium nanoparticles (Ru-NP) for the production of valuable chemicals. Due to its demonstrated durability, the catalyst could be widely used in the large-scale production of many types of dyes, detergents, agrochemicals and pharmaceuticals.
A study led by researchers at Tokyo Institute of Technology (Tokyo Tech) has shown that nanoscale changes in surface structure can vastly improve the performance of metal catalysts used in the production of primary amines, an important class of compounds in the chemical industry.
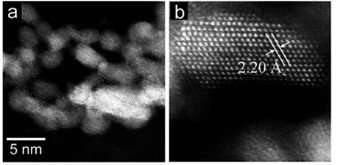
The researchers developed a ruthenium-based catalyst composed of a large number of atomically active facets on their flat surfaces.
Compared to conventional metal-supported catalysts, the new catalyst, with its highly active surface structure, achieved an overwhelmingly high catalytic turnover.
The study involved comparing how well different kinds of metal catalysts could convert biomass-derived furfural to furfurylamine. This served as a model reaction for reductive amination, the main process used to yield primary amines.
The new catalyst exhibited a turnover frequency of 1850 per hour. This figure represents a six-fold increase in efficiency over a metal-supported catalyst (RU/NB2O5) developed previously by some of the same team members, including Michikazu Hara and Keigo Kamata at Tokyo Tech's Institute of Innovative Research.
"To the best of our knowledge, the Ru-NP catalyst exhibited the highest turnover frequency among the metal catalysts reported to date for the reductive amination of furfural to produce furfurylamine," the team writes in their study published in Chemical Science.
Further experiments to test the reusability of the new catalyst for the reductive amination of furfural showed "no noticeable decrease in activity, even after four reuses," the researchers say, highlighting the catalyst's robustness.
The secret behind the catalyst's exceptional performance lies in its distinctive structure and shape. Usually, Ru has a hexagonal close-packed structure that limits catalytic performance. In contrast, the new catalyst has a high proportion of so-called flat-shaped, pristine face-centered-cubic ruthenium nanoparticles at the surface, which serve as active sites with weak electron-donating ability. It is this feature that is thought to be critical to enabling efficient and selective reductive amination of various carbonyl compounds.
As well as facilitating the production of biomass-derived amines, the new catalyst could also help meet the demands of the wider fine chemical industry.
More information: Debraj Chandra et al, A high performance catalyst of shape-specific ruthenium nanoparticles for production of primary amines by reductive amination of carbonyl compounds, Chemical Science (2018). DOI: 10.1039/c8sc01197d
Journal information: Chemical Science
Provided by Tokyo Institute of Technology