The sweet taste of success for a supported nickel phosphide nanoalloy catalyst
Catalysts lie at the heart of a greener and more sustainable future for chemical production. However, many of the catalysts currently in widespread use have limitations that affect their efficiency. Researchers from Osaka University have reported a stable and reusable nickel phosphide nanoalloy catalyst for the hydrogenation of maltose to maltitol that outperforms conventional catalysts. Their findings are published in ACS Sustainable Chemistry & Engineering.
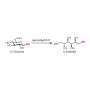
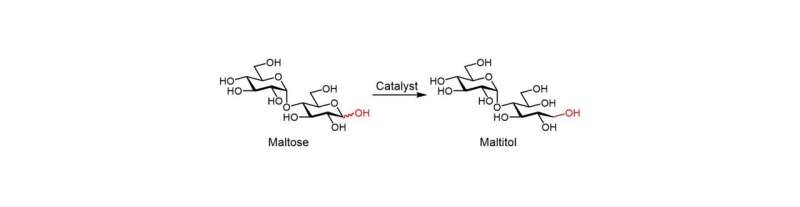
Maltitol is a sugar alcohol that is widely used as a sweetener and food additive. It can be produced by hydrogenating maltose; however, the reaction must be selective to avoid generating unwanted side products such as glucose. Ruthenium catalysts have been found to be efficient for this conversion, but are expensive, while cheaper nickel alternatives have low activity and are difficult to handle and reuse.
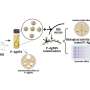
The researchers have now reported a nickel phosphide nanoalloy catalyst on a hydrotalcite (HT) support (nano-Ni2P/HT) that shows high activity for the selective hydrogenation of maltose to maltitol. The catalyst is also stable in air making it easy to handle.
"Our catalyst outperformed conventional catalysts for maltitol synthesis, showing high activity even at ambient temperature," says study first author Sho Yamaguchi. "The HT support was found to be key to the enhanced performance. In fact, the turnover number of the supported catalyst was more than 300 times higher than that of the same catalyst without a support."
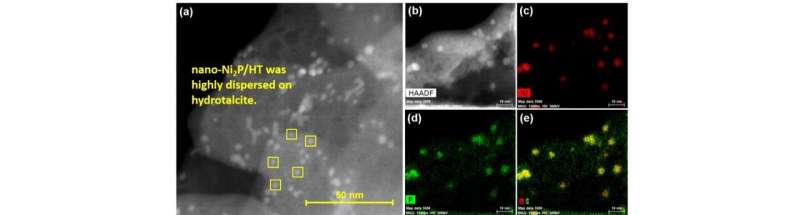
The catalyst and support were found to work together in so-called cooperative catalysis. The nickel sites on the nano-Ni2P are thought to activate the hydrogen gas, while the HT is believed to be an electron donor and to activate the maltose.
nano-Ni2P/HT could be filtered from the reaction mixture and reused directly, without the need for time-consuming regeneration steps. The same amount of maltitol was produced on the fifth use as when the catalyst was fresh, showing that the activity and selectivity were conserved after multiple uses.
The catalyst even achieved high yields when the reaction mixture had a high maltose concentration (>50 wt%), which indicates that it would be appropriate for use on an industrial scale.
"The cooperative role of the support in the high activity of nano-Ni2P/HT is particularly exciting because this area has not been widely explored," study corresponding author Takato Mitsudome explains. "We believe that this mechanism, supported by the excellent properties we have demonstrated, means our catalyst is perfectly positioned to make a significant contribution to the sustainable production of maltitol."
More information: Sho Yamaguchi et al. Support-Boosted Nickel Phosphide Nanoalloy Catalysis in the Selective Hydrogenation of Maltose to Maltitol ACS Sustainable Chemistry & Engineering April 20, 2021 DOI: 10.1021/acssuschemeng.1c00447
Provided by Osaka University