Coordination polymer glass provides solid support for hydrogen fuel cells

Scientists at Japan's Institute for Integrated Cell-Material Sciences (iCeMS) are leading efforts to synthesize stronger and efficient materials for hydrogen fuel cell membranes. Most fuel cells currently on the market employ liquid membranes. A new coordination polymer glass membrane, reported in the journal Chemical Science, works just as well as its liquid counterparts with added strength and flexibility.
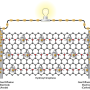
Hydrogen fuel cells are fed hydrogen and oxygen to produce electricity, with water as their only by-product. These fuel cells contain 'proton conducting membranes' that facilitate the separation of hydrogen's positive and negative particles, protons and electrons, a process that ultimately leads to the production of electricity.
Protons need to easily move across these membranes for the process to be efficient. Current proton conducting membranes are made from liquids and cannot operate effectively under dry conditions, making their fabrication complicated and expensive. Scientists are looking for ways to fabricate solid membranes made from water-free electrolytes that provide better mechanical and thermal stability than their liquid counterparts, but are also cost-effective and still conduct protons well.
"Our coordination polymer glass performed better than recently reported ionic liquids and crystalline coordination polymers," says Satoshi Horike, a materials scientist at Kyoto University's Institute for Integrated Cell-Material Sciences (iCeMS) who led the research.
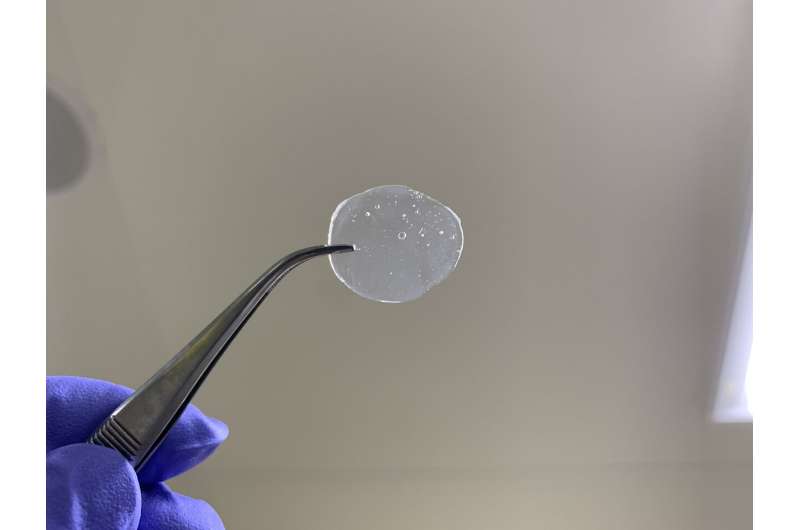
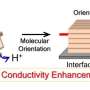
Horike, Tomohiro Ogawa and colleagues in Japan fabricated their coordination polymer glass membrane by mixing a 'protic ionic liquid' with zinc ions. Protic ionic liquids are liquid salts made by mixing an acid and a base. The team used a protic ionic liquid called diethylmethylammonium dihydrogen phosphate. Adding zinc to this liquid led to the formation of a solid, elastic polymer glass.
The molecular structure of the coordination polymer glass facilitated the movement of protons across it under dry conditions at 120°C. When tested in a hydrogen fuel cell, it produced high voltage (0.96 volts), well within the range of typical polymer electrolyte membranes. Its power output was also similar to commonly used Nafion membranes.
Ogawa believes their findings offer an interesting approach for using glass polymers in fuel cell applications. The team plans to continue their work with the aim of achieving fuel cell membranes with higher performance and long-term stability.
More information: Tomohiro Ogawa et al, Coordination Polymer Glass from Protic Ionic Liquid: Proton Conductivity and Mechanical Property as Electrolyte, Chemical Science (2020). DOI: 10.1039/d0sc01737j
Journal information: Chemical Science
Provided by Kyoto University