Salt takes a quick step before falling out of water

When a drop of sea spray lands on a rock and heats under the midday sun, the salt crystalizes and falls out of the evaporating water as a crystal—helping to power the Earth's atmosphere and leaving a delicious kernel of spice for dinner.
Now, in a finding with implications for everything from climate models to drug production, computational research from Princeton University has shown that the process includes an extra step.
"We were trying to understand how solids fall out of solution as crystals," said Athanassios Panagiotopoulos, the Susan Dod Brown Professor of Chemical and Biological Engineering and the project's lead researcher. "This happens when you are trying to make a pharmaceutical component, to get your desired ingredient in pure form. It is also relevant for atmospheric processes. So, both for environmental applications and technological applications, this is a very important process."
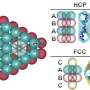
The results, published as a cover article in the Journal of Chemical Physics on March 22, show that when salts in a supersaturated solution precipitate as crystals, they first go through a brief intermediate phase. In this first quick step, salt ions in the solution form disordered clusters that the researchers have called "amorphous salt," which represent a semi-crystalline state. That state lasts between 10 and 100 nanoseconds, mere billionths of a second, before the semi-crystals begin to rearrange themselves into a more ordered state as true crystals.
The work required complex computational models running for several months to see how these solutions evolve beyond their saturation thresholds. The researchers believe this new framework will allow scientists to have a better, more accurate framework for their experimental results.
In addition to Panagiotopoulos, chair of chemical and biological engineering, the team included Hao Jiang, formerly a postdoctoral researcher in chemical and biological engineering and now at the University of Pennsylvania; and Pablo Debenedetti, the Class of 1950 Professor in Engineering and Applied Science and Princeton's Dean for Research.
More information: Hao Jiang et al. Nucleation in aqueous NaCl solutions shifts from 1-step to 2-step mechanism on crossing the spinodal, The Journal of Chemical Physics (2019). DOI: 10.1063/1.5084248
Journal information: Journal of Chemical Physics
Provided by Princeton University