Chemists synthesize a new hybrid organic-inorganic catalyst
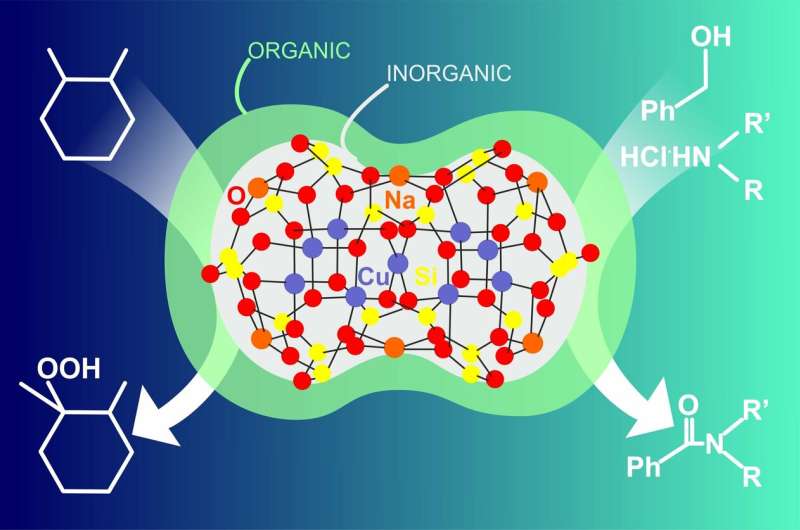
RUDN chemists have synthesized metal complexes on the basis of the organoelemental substance silsesquioxane that consists of an organic and an inorganic part. Such hybrid systems may be used as efficient catalysts, for example, to obtain alcohols from alkanes. The work was published in the Inorganic Chemistry journal.
Physical and chemical parameters of any material or substance are limited and cannot be infinitely improved. So scientists work on hybrid materials that combine different components and therefore demonstrate new properties. In modern chemistry, special attention is paid to compounds that consist of metal centers and organic "bridges" that keep them together. Such objects have a number of valuable properties and may be used for industrial purposes: catalysis, storage of gases, accurate separation of mixed substances. They can also be used to create chemical sensors and agents to deliver drugs to their targets in the body.
Hybrid organoelemental substances such as silsesquioxanes consist of an inorganic main chain Si-O-Si and an organic framework of Si atoms. Compounds like this can be formed when metal atoms are added to carcass structures with promising catalytic and magnetic properties. RUDN chemists suggested a new approach to such compounds based on the use of additional complex-forming substances (ligands).
The new products were obtained in the course of a silsesquioxane and copper dichloride self-assembly reaction in the presence of organic ligands—phenanthroline and neocuproine. In the first case the scientists observed the case of "hidden control" as phenanthroline facilitated the formation of a previously unknown carcass compound but was not included into the product. The use of the second ligand led to an unusual result: the product consisted of several components with copper atoms distributed among ligands of different nature—an oxygen-consisting one (silsesquioxane) and a nitrogen-consisting one (neocuproine). The first obtained substance was used in oxidizing reactions of organic synthesis—amidation and functionalization of alkanes and alcohols.
"We managed to obtain unusual objects with high concentration of metal centers (in this case—copper atoms) incorporated into a silicon-organic matrix. To carry out such reactions we used various organic ligands changing the standard mechanisms of carcass formation. We studied the obtained substances as catalysts of chemical reactions, and their catalyst activity in the amidation reaction turned out to remain quite high even at very low concentrations (100 ppm in copper)," said Alexey Bilyachenko, a co-author of the work and Deputy Director of the United Institute of Chemical Research, RUDN.
More information: Grigorii S. Astakhov et al. High-Cluster (Cu9) Cage Silsesquioxanes: Synthesis, Structure, and Catalytic Activity, Inorganic Chemistry (2018). DOI: 10.1021/acs.inorgchem.8b01496
Journal information: Inorganic Chemistry
Provided by RUDN University