Scientists suggest an eco-friendly way of obtaining highly active catalysts
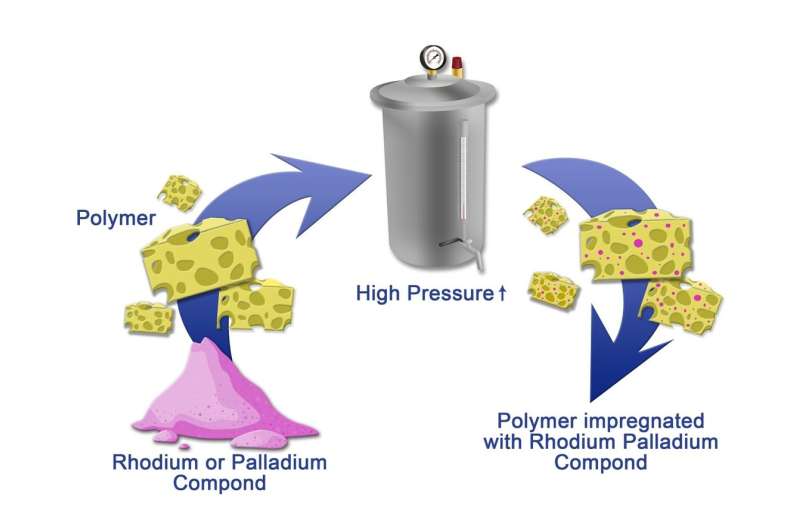
A team from Sechenov University and Russian colleagues developed a method for obtaining substances that accelerate the binding of hydrogen molecules with hydrocarbons via double bonds. The peculiarity of this approach lies in the impregnation of a polymeric carcass with rhodium- and palladium-containing salts in the supercritical CO2 environment. The latter is an eco-friendly alternative of traditional organic solvents. The work was published in The Journal of Supercritical Fluids.
Modern-day solid catalysts (compositions that accelerate chemical reactions) mainly consist of a porous polymer with a metal or its oxide on the surface. A traditional way of creating such composites includes the impregnation of the carcass with a metal-containing substance in the environment of organic solvents. Recently, chemists have been focusing on the development of new methods, including those based on the use of supercritical liquid CO2 (at the temperature over 31° and pressure above 73 atmospheres). This system has the characteristics of both a liquid and a gas, and the environment is able to solve a number of non-organic substances as well as to secure their fast movement in the mixture. Compared to organic solvents, supercritical CO2 is safe, cheap, and easy to remove.
"Currently, there are few studies covering the synthesis of metal-polymeric catalysts in the environment of supercritical carbon dioxide. In our work, we showed the efficiency of this method and demonstrated the high activity of the obtained substances in the reaction of hydrogen combination with organic molecules with double bonds (i.e. their hydrogenation)," says Petr Timashev, Ph.D. in chemistry, director of the Institute of Regenerative Medicine at I.M. Sechenov First Moscow State Medical University.
Chemists from MSMU synthesized several types of catalysts using porous phenol-formaldehyde resin (an amorphous substance) or branched nitrogen containing polymers as a carcass. Having treated them with palladium and rhodium salts of organic acid in the presence of supercritical CO2, the scientists obtained samples with metal nanoparticles. Experiments showed high activity of all types of the catalysts. It can also be adjusted by changing the conditions of the synthesis. For example, the longer the time of metal impregnation with salt occured, the bigger the product yield of the reaction is. Moreover, the new catalysts are especially selective in combination with olefins—hydrocarbons with one double bond. This property may come useful for the modification of vegetable oils: fats obtained during this procedure are widely used in baking and confectionary industries, as well as in the synthesis of pheromones and aromatic substances.
"Besides the quality of the obtained catalysts, this approach is advantageous from the ecological point of view. The majority of currently used organic solvents are toxic. This is not true for carbon dioxide," concludes Petr Timashev.
More information: Oleg P. Parenago et al, Obtaining of highly-active catalysts of unsaturated compounds hydrogenation by using supercritical carbon dioxide, The Journal of Supercritical Fluids (2018). DOI: 10.1016/j.supflu.2018.07.010
Provided by Sechenov University



















