Super cheap earth element to advance new battery tech to the industry
Most of today's batteries are made up of rare lithium mined from the mountains of South America. If the world depletes this source, then battery production could stagnate.
Sodium is a very cheap and earth-abundant alternative to using lithium-ion batteries that is also known to turn purple and combust if exposed to water—even just water in the air.
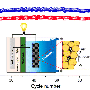
Worldwide efforts to make sodium-ion batteries just as functional as lithium-ion batteries have long since controlled sodium's tendency to explode, but not yet resolved how to prevent sodium-ions from "getting lost" during the first few times a battery charges and discharges. Now, Purdue University researchers made a sodium powder version that fixes this problem and holds a charge properly.
"Adding fabricated sodium powder during electrode processing requires only slight modifications to the battery production process," said Vilas Pol, Purdue associate professor of chemical engineering. "This is one potential way to progress sodium-ion battery technology to the industry."
The study was made available online in June 2018 ahead of print on August 31, 2018 in the Journal of Power Sources.
This work aligns with Purdue's giant leaps celebration, acknowledging the university's global advancements made in health, space, artificial intelligence and sustainability as part of Purdue's 150th anniversary. Those are the four themes of the yearlong celebration's Ideas Festival, designed to showcase Purdue as an intellectual center solving real-world issues.

Even though sodium-ion batteries would be physically heavier than lithium-ion technology, researchers have been investigating sodium-ion batteries because they could store energy for large solar and wind power facilities at lower cost.
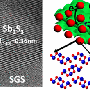
The problem is that sodium ions stick to the hard carbon end of a battery, called an anode, during the initial charging cycles and not travel over to the cathode end. The ions build up into a structure called a "solid electrolyte interface."
"Normally the solid electrolyte interface is good because it protects carbon particles from a battery's acidic electrolyte, where electricity is conducted," Pol said. "But too much of the interface consumes the sodium ions that we need for charging the battery."
Purdue researchers proposed using sodium as a powder, which provides the required amount of sodium for the solid electrolyte interface to protect carbon, but doesn't build up in a way that it consumes sodium ions.
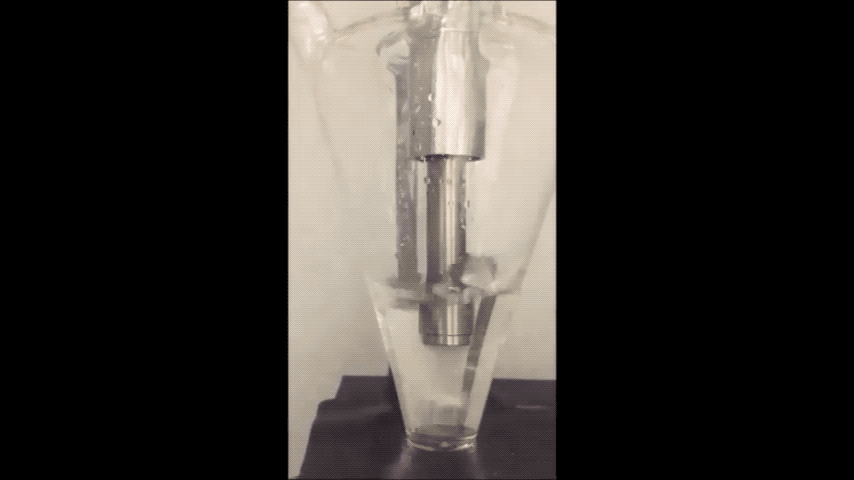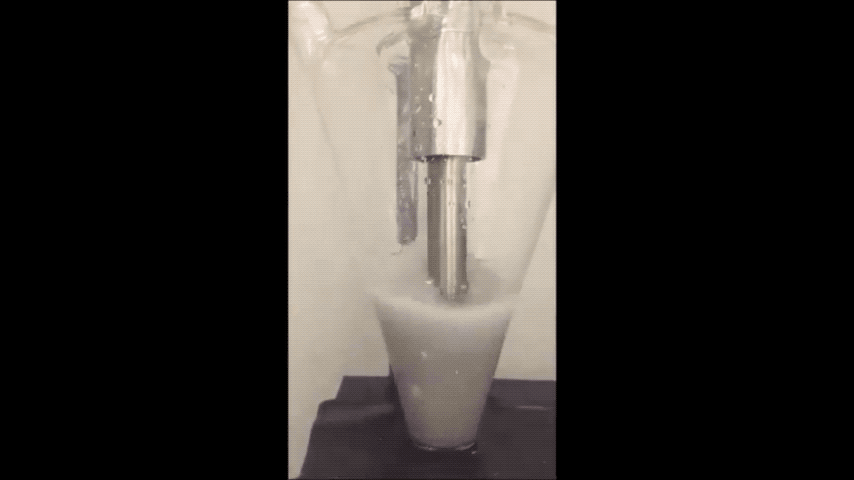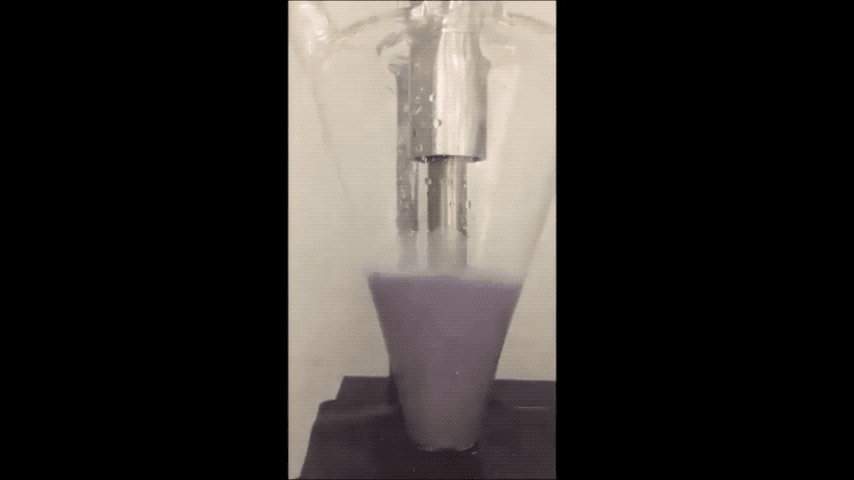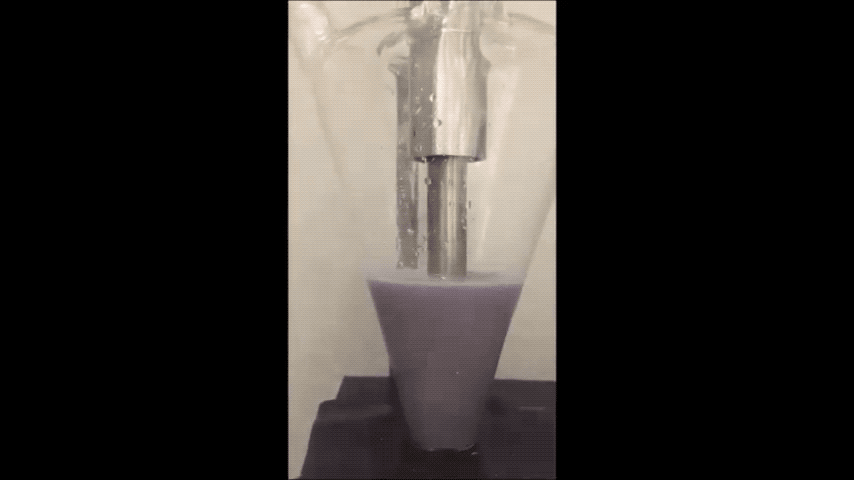
They minimized sodium's exposure to the moisture that would make it combust by making the sodium powder in a glovebox filled with the gas argon. To make the powder, they used an ultrasound—the same tool used for monitoring the development a fetus—to melt sodium chunks into a milky purple liquid. The liquid then cooled into a powder, and was suspended in a hexane solution to evenly disperse the powder particles.
Just a few drops of the sodium suspension onto the anode or cathode electrodes during their fabrication allows a sodium-ion battery cell to charge and discharge with more stability and at higher capacity—the minimum requirements for a functional battery.
More information: Jialiang Tang et al, Ultrasound-assisted synthesis of sodium powder as electrode additive to improve cycling performance of sodium-ion batteries, Journal of Power Sources (2018). DOI: 10.1016/j.jpowsour.2018.06.067
Provided by Purdue University